MIT chemical engineers have made a groundbreaking discovery that could turn a major environmental villain—carbon dioxide (CO2)—into valuable products like fuels and chemicals.
This innovative method converts CO2 into carbon monoxide (CO), a critical ingredient for creating things like ethanol, a type of alcohol used in beverages and as fuel, among other useful compounds.
Carbon dioxide is a significant contributor to the greenhouse effect, trapping heat in the Earth’s atmosphere and leading to global warming.
This new technology offers a promising solution by potentially allowing us to remove CO2 from places where it’s usually released in large amounts, such as power plants, and turn it into something useful instead of letting it warm our planet.
Ariel Furst, a leading mind behind this research at MIT, is excited about the possibilities. “Imagine taking carbon dioxide from factories or even the ocean and turning it into valuable chemicals,” she says.
“This could be a big step towards reducing our carbon footprint and combating climate change.”
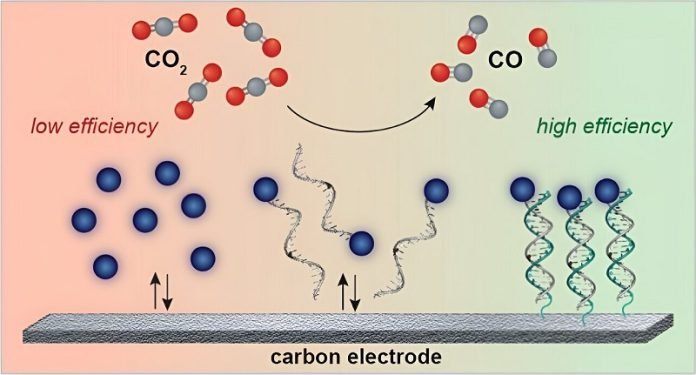
The secret to their success lies in an ingenious use of electricity and a specially designed catalyst—a substance that speeds up a chemical reaction without being used up in the process.
But there’s a twist: this catalyst is attached directly to the electrode (a conductor through which electricity enters or leaves) with the help of DNA.
Yes, you read that right—DNA, the same molecule that carries genetic information in living organisms, is used here as a kind of molecular Velcro, keeping everything needed for the reaction close together.
This setup significantly enhances the efficiency of the process.
Previous attempts to turn CO2 into CO faced challenges, mainly because it took a lot of energy, making the process too costly.
By employing a type of molecule called porphyrins, which are similar to the ones in our blood that transport oxygen, along with their innovative DNA technique, the MIT team was able to make this conversion far more energy-efficient and practical.
During the process, carbon dioxide is dissolved in water within a special device. Then, when electricity is applied, the catalyst on the electrode gets to work, transforming CO2 into CO and a small amount of hydrogen gas.
The really cool part is that once the catalysts have done their job, they can be easily replaced with new ones by simply heating the system, thanks to the reversible nature of the DNA bonds.
The efficiency of this new method is impressive. Normally, a lot of the electrical energy used in reactions like this goes to waste.
But with their approach, the MIT researchers achieved a 100% Faradaic efficiency, meaning all the energy put into the system is used for the conversion, with no loss.
This technology could be a game-changer for reducing greenhouse gas emissions on a large scale, especially since the materials needed, like carbon electrodes and the catalysts, are relatively inexpensive and don’t require rare metals.
The team is looking into using this method to produce other products, such as methanol and ethanol, expanding the potential applications.
Ariel Furst has even started a company, Helix Carbon, to continue developing this technology for commercial use.
This breakthrough represents a hopeful path towards using our knowledge and technology to fight climate change by recycling one of its primary causes into something useful.



