In a major advancement from The University of Texas at Austin, researchers have developed a new type of sodium battery that is not only safer but also uses cheaper and more common materials.
This development is crucial as the world uses more batteries, leading to a higher chance of battery fires.
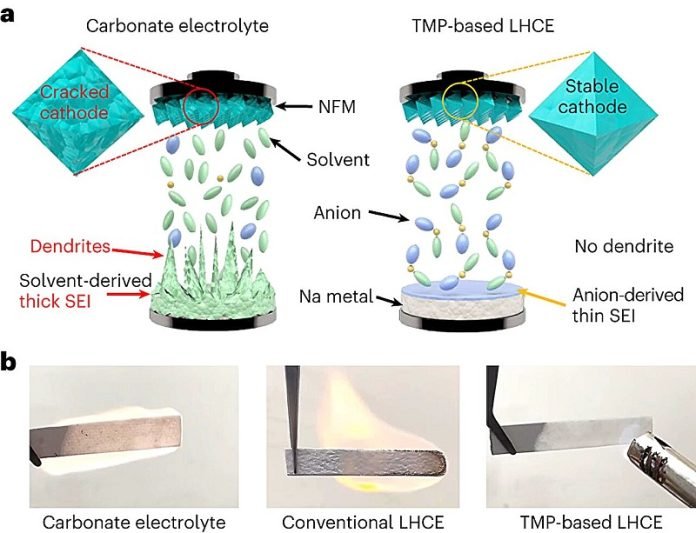
The key to making this sodium battery safer lies in a special ingredient added to the electrolyte—the part of the battery that moves charge between the two ends.
This ingredient, a type of salt called sodium nitrate, allows the battery to use a single, nonflammable liquid, making the whole battery much more stable.
Usually, batteries can become dangerous over time. The liquids inside them can react with other parts, wearing the battery down and sometimes causing fires or even explosions. This is particularly a challenge with sodium, which is very reactive. The new battery avoids these dangers, offering a much-needed solution.
Arumugam Manthiram, a leading researcher on the project, explains, “Batteries catch fire because the liquid inside them reacts badly with other battery parts. We’ve found a way to remove this danger, creating a safer and more stable battery.”
What’s more, this sodium battery could be a game-changer in reducing costs. It’s designed to be an alternative to lithium-ion batteries, which are used in everything from smartphones to electric cars but are expensive and have environmental downsides.
The performance of this new battery is impressive. It keeps 80% of its energy capacity even after being used and recharged 500 times. This is comparable to the lifespan of lithium-ion batteries found in smartphones.
Manthiram highlights the importance of their work, saying, “We’ve made a sodium battery that’s not only safe and cheap but also doesn’t compromise on how well it works. It’s crucial we find alternatives to lithium-ion batteries that are even better.”
Although this breakthrough is with a sodium battery, the researchers believe the technique could also be applied to lithium-ion batteries with some adjustments in materials.
One of the reasons this development is so important is the environmental and economic impact of lithium mining, which is expensive and harmful to the environment. Sodium, on the other hand, is plentiful in the ocean, making it a cheaper and more eco-friendly option.
Lithium-ion batteries also typically use cobalt, a material that’s not only costly but also has significant environmental and health impacts, especially in places like the Democratic Republic of the Congo where it’s mainly mined. The team, led by Manthiram, has previously developed a lithium-ion battery without cobalt. This new sodium battery goes a step further by avoiding cobalt and lithium altogether, using materials that are 40% iron, 30% manganese, and 30% nickel instead.
This groundbreaking work was a team effort, with contributions from researchers at the Cockrell School’s Materials Science and Engineering program and Texas Materials Institute, as well as John Okasinski from the Argonne National Laboratory.
This sodium battery represents a significant step forward in making energy storage safer, more affordable, and more sustainable.
It opens up new possibilities for powering our devices and vehicles in the future, without the risks and costs associated with current battery technology.



