In the quest for cleaner and more efficient energy storage, scientists are diving deep into the world of batteries, exploring every possible way to make them better.
At the heart of this exploration are sodium-ion batteries, a promising alternative to the lithium-ion batteries that power everything from our smartphones to electric cars.
However, sodium-ion batteries have their own set of challenges that need addressing before they can take center stage.
Enter a dedicated team from the Helmholtz-Zentrum Berlin (HZB) and Humboldt University of Berlin, who’ve embarked on an intriguing scientific adventure.
Their mission? To understand how tweaking the battery’s cathode material with tiny bits of other elements—a process known as “doping”—can dramatically improve the battery’s performance.
Their findings, published in the journal Advanced Materials, shed new light on this complex puzzle.
Why Sodium-ion?
Lithium-ion batteries are the champions of energy storage, boasting the highest energy density (energy stored per kilogram) available. But there’s a catch: lithium is not only scarce but also expensive.
Sodium, on the other hand, is like the sand at the beach—plentiful and cheap. It doesn’t store quite as much energy as lithium, but for many applications, like storing solar energy for a rainy day, it’s just the ticket.
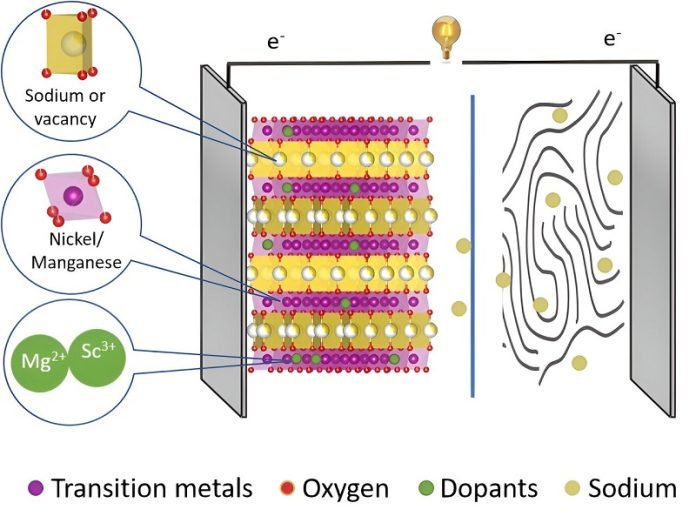
The team focused on the cathode part of the battery, where electricity goes out during use. This part is usually made from layers of metal oxides, with spaces in between where sodium ions snuggle in and out as the battery charges and discharges.
But there’s a problem: sometimes, the cathode material reacts in ways that can shorten the battery’s life.
To tackle this, the researchers experimented with adding tiny amounts of scandium and magnesium to the cathode material. These elements are sort of like the new kids on the block, slightly different in character but able to blend in, potentially stabilizing the cathode’s structure and improving the battery’s lifespan and capacity.
X-Rays Reveal Secrets
The investigation wasn’t simple. It took three years and visits to three different X-ray facilities: BESSY II, PETRA III, and SOLARIS. Each of these places offered unique tools to peek inside the batteries in ways that are not possible with regular equipment.
They used techniques like resonant inelastic X-ray scattering and X-ray absorption spectroscopy to see how the cathode’s structure changed with doping, and X-ray diffraction to understand its overall structure.
What they found was a bit of a twist. Adding scandium didn’t make the cathode more stable as expected. Meanwhile, magnesium doping had an unexpected benefit: it tamped down an unwanted reaction involving oxygen that could degrade the battery over time.
This reaction was minimized when the amount of magnesium added was just right, striking a delicate balance that enhanced the battery’s performance.
This research is more than a scientific curiosity. It’s a step toward making sodium-ion batteries a viable option for a wide range of uses, from powering homes to storing renewable energy.
The team’s work shows that the secret to better batteries might not just be in choosing the right materials but in fine-tuning their composition with precision.
As we look to a future powered by clean energy, these discoveries offer a glimpse of the innovative approaches that will help us get there.



