Batteries are everywhere in our daily lives, from our smartphones to electric cars. The University of Texas at Austin has recently made a big leap in making these batteries even better.
Imagine the battery in your phone or car. It has several parts, but one of the main ones is called the electrode.
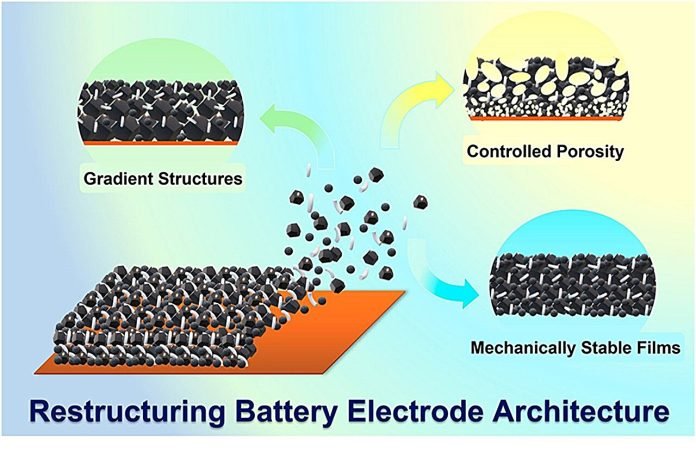
The researchers at UT Austin have come up with new ways to build these electrodes. They’re like the chefs of the battery world, mixing up new recipes for better performance.
One of their cool ideas is making the insides of the battery like a sponge with lots of tiny holes, or pores.
This helps the battery charge and discharge faster, which means your phone could charge quicker or your electric car could go longer without needing a plug-in.
They’ve also created a kind of gradient design in the electrodes. Think of it like a layered cake, where each layer has a different flavor. In the battery, each layer helps store and transfer energy in the most efficient way possible.
Another big change is getting rid of something called the metal foil current collector. This part is usually needed in batteries, but by removing it, the team made the battery more stable and able to hold more energy.
This could lead to lighter and smaller batteries that still pack a powerful punch.
The UT Austin team is not just thinking about making these batteries stronger. They know it’s important to be able to make them on a large scale without it costing a fortune. This way, these better batteries can actually get into our hands and not just stay in the lab.
Professor C. Buddie Mullins, a key member of the research team, is excited about what this means for the future. He believes these new battery designs can help us move towards more reliable and sustainable ways to store energy.
This could be a big deal for everything from electric cars to storing energy from sources like wind and solar power.
But that’s not all. There’s another part of the battery called the electrolyte, and it’s like the medium that helps energy flow inside the battery. The UT Austin team has also been working on making better electrolytes.
They’re experimenting with different types, like ones that work well even in really cold temperatures. This means batteries that are more reliable in different conditions.
In simple terms, the team at UT Austin is working on making batteries that can store more energy, charge faster, last longer, and work in a wider range of temperatures.
This could have a big impact on things like electric vehicles, renewable energy storage, and all the portable gadgets we use every day.
It’s a step forward in meeting our growing need for energy in a way that’s more efficient and adaptable.



