The vast oceans, covering most of Earth, are not just home to countless lifeforms but also a potential source of sustainable nuclear fuel.
Hidden in the waters are tiny amounts of uranium ions, which, if extracted efficiently, could power our future with nuclear energy.
In a significant development, researchers have introduced a new material for extracting uranium from seawater, as reported in ACS Central Science.
This breakthrough could change the way we think about nuclear power.
Nuclear power plants use a process called fission, where atoms are split apart to release energy in the form of heat and electricity. Uranium is the preferred element for this because it’s naturally unstable and radioactive, making it easier to break apart.
Currently, uranium is mined from rocks on land. However, these resources are limited. In contrast, according to the Nuclear Energy Agency, our oceans hold over 1,000 times more uranium, dissolved as uranyl ions than what’s found on land – about 4.5 billion tons.
Getting uranium out of seawater has been a tough task. The main hurdle is that existing materials used for this purpose don’t have enough surface area to capture the uranium ions effectively.
This is where the new research led by Rui Zhao and Guangshan Zhu comes into play. They aimed to create a material that could efficiently trap these elusive ions.
The team started with a flexible cloth made of carbon fibers. This cloth was then coated with specialized chemicals and treated to add amidoxime groups to the polymers.
Amidoxime is known for its ability to latch onto uranium ions. The cloth’s naturally porous structure was perfect for creating lots of tiny spaces where the amidoxime could snuggle in and grab the uranium ions from seawater.
Testing the New Material
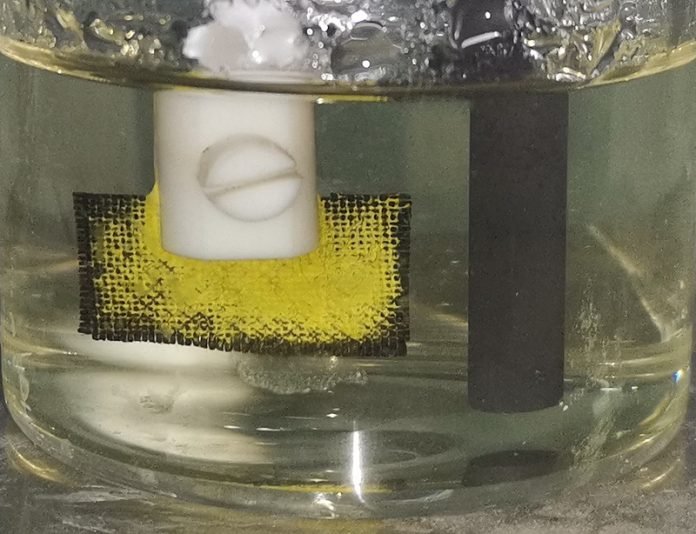
To test their invention, the researchers placed the coated cloth as one electrode (the cathode) in seawater, with a graphite rod serving as the other electrode (the anode). They then ran a current between these two points. Over time, they observed bright yellow uranium-based deposits forming on the cathode cloth.
In real-world conditions, using seawater from the Bohai Sea, their method extracted 12.6 milligrams of uranium per gram of water over 24 days. This efficiency was higher than many other materials tested by the team.
Moreover, using this electrochemical method was about three times faster than letting the uranium ions accumulate naturally on the cloths.
This research presents a promising way to harvest uranium from our oceans. If this method can be scaled up and made cost-effective, it could turn the vast waters of our planet into a significant source of nuclear fuel.
This could be a game-changer, offering a more sustainable and abundant source of uranium for nuclear power, which is a clean and efficient form of energy.
As we continue to seek solutions for our growing energy needs and environmental challenges, tapping into the ocean’s uranium reserves could be an important part of the puzzle.



