In a significant scientific advancement, researchers at the RIKEN Cluster for Pioneering Research in Japan, led by Genki Kobayashi, have created a new material that could revolutionize hydrogen-based batteries and fuel cells.
This development, detailed in the journal Advanced Energy Materials, brings us closer to harnessing the full potential of hydrogen energy, which is safer, more efficient, and packs more power.
Hydrogen energy is seen as a key player in our future energy needs, but there have been hurdles in making it widely practical and efficient.
Presently, hydrogen-based fuel cells, like those in electric cars, work by moving hydrogen protons across a fuel cell through a polymer membrane.
This process, while effective, needs constant hydration to prevent the membrane from drying out, adding complexity and cost to the system.
The breakthrough by Kobayashi’s team lies in their development of a solid electrolyte that conducts hydride ions (H−) at room temperature. Hydride ions are negatively charged hydrogen atoms, and moving them efficiently at normal temperatures has been a significant challenge until now.
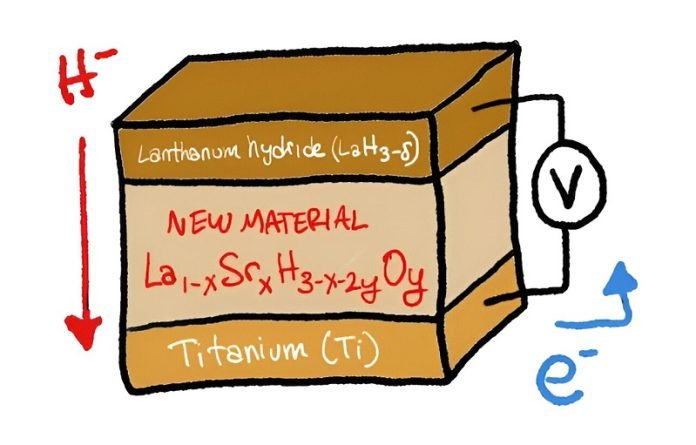
The researchers experimented with a compound known as lanthanum hydrides (LaH3-δ). This material is attractive because it can easily release and capture hydrogen, and it works effectively at temperatures below 100°C.
However, at room temperature, it faced a problem. The amount of hydrogen attached to the lanthanum fluctuates, which hindered efficient ion conduction. This issue, known as hydrogen non-stoichiometry, was the major obstacle the team had to overcome.
Their solution was innovative. By substituting some of the lanthanum with strontium (Sr) and adding a small amount of oxygen, they created a new formula: La1-xSrxH3-x-2yOy. This composition allowed for efficient hydride ion conduction at room temperature.
To test their discovery, the team prepared crystalline samples of this new material through a process called ball-milling, followed by annealing. They found that at room temperature, their material conducted hydride ions at an impressive rate.
They then used this material in a solid-state fuel cell combined with titanium and observed that with the right amount of strontium (at least 0.2), the conversion of titanium to titanium hydride (TiH2) was complete. This meant that there was almost no loss of hydride ions in the process.
Kobayashi sees this as a milestone in the field. “Our results provide material design guidelines for hydride ion-conducting solid electrolytes,” he states. Looking forward, this discovery is not just a step but a leap in the development of batteries, fuel cells, and electrolytic cells that use hydrogen.
The next stage of their research will focus on enhancing performance and developing electrode materials that can repeatedly absorb and release hydrogen. This would allow for rechargeable batteries and more efficient hydrogen storage and release, crucial for widespread hydrogen energy use.
In essence, this breakthrough marks a turning point in our journey towards a practical hydrogen-based energy economy, promising a future with safer, more efficient, and higher-density energy solutions.