In the world of batteries, lithium-ion has been king, but it’s not without its problems.
One major issue is that lithium, a key ingredient, isn’t easy to get.
This is where a team of engineers from the University of Maryland is making waves with a new kind of battery that uses sodium, a much more common and sustainable element.
Sodium is everywhere, especially in seawater.
That makes it a very attractive option for batteries. Not only is it abundant, but it’s also potentially cheaper than lithium, which is a big deal considering our growing need for energy storage.
The sodium-ion batteries we have right now use a liquid to transfer charge, but this liquid can catch fire. That’s a big risk, especially for larger batteries in things like electric cars or storage for solar panels.
The team at the University of Maryland has developed a new kind of sodium battery that solves this problem.
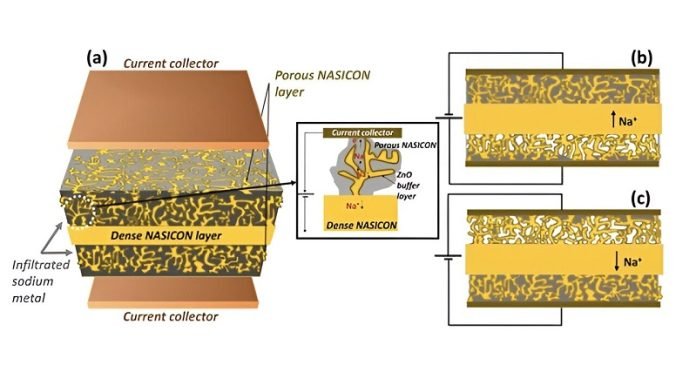
They used a special material called Sodium (Na) Super Ionic Conductor (NASICON). NASICON is solid, not liquid, which means it doesn’t have the same fire risk. Plus, it’s really good at moving sodium ions around inside the battery, which is key for storing and releasing energy.
This new battery is called a solid-state sodium battery (SSSB).
It’s different from other sodium-ion batteries because it uses solid NASICON as an electrolyte – the part of the battery that ions move through.
This makes it safer and more stable. It also uses sodium metal for the anode (one of the battery’s electrodes), which helps it store more energy.
Dr. Eric Wachsman from the University of Maryland explained how important this is. Until now, no one has been able to make a solid-state sodium battery work this well at room temperature.
This new design can handle a lot of charging and discharging cycles at high speeds without losing performance. That’s a game-changer for solid-state sodium batteries.
The research, published in the journal Energy & Environmental Science, shows a lot of promise for high-energy, safe, and cost-effective batteries.
The team’s success in making these batteries cycle efficiently at high currents and with thin NASICON structures is a major step towards making better energy storage systems.
With this advancement, we could see sodium batteries becoming a big player in the world of energy storage.
They could be used in everything from electric cars to storing energy from renewable sources like solar and wind.
And since sodium is so common, these batteries could be a lot cheaper and more sustainable than the lithium-ion batteries we currently rely on.
In summary, the University of Maryland’s development of a high-performing, safer, and potentially cheaper sodium battery is an exciting step forward.
It opens the door to more sustainable energy storage options, which is crucial as we move towards a future powered by renewable energy.