When it comes to finding the secret to batteries that last longer, scientists may have found an unlikely inspiration: the humble bar of soap.
Researchers from Brown University and Idaho National Laboratory have discovered that the way soap cleans might hold clues to creating batteries that not only store more energy but also last for more charges than ever before.
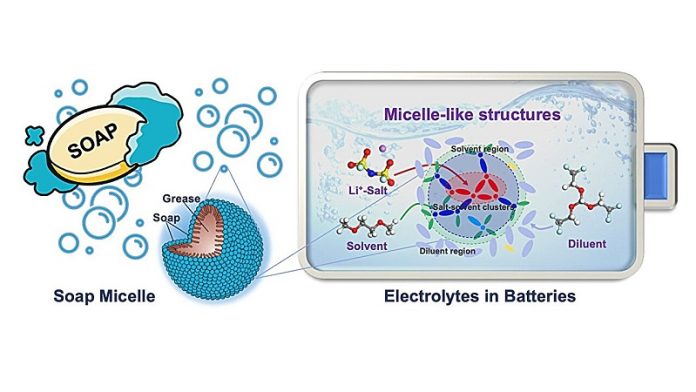
Here’s a fun fact about soap: when you wash your hands, tiny structures in the soap, called micelles, grab onto the oil and dirt, wrapping them up so they can be rinsed away with water.
Soap is like a mediator that brings together water and dirt, allowing them to mix and be washed off your skin.
This everyday act of handwashing has sparked an idea in the minds of battery engineers.
In a recent study, featured in the prestigious journal Nature Materials, they’ve looked at a similar kind of interaction within a new type of electrolyte—a key component in batteries, especially the kinds we want to use in lithium metal batteries.
These are the next big thing in battery tech because they have the potential to store way more energy than the lithium-ion batteries we use today.
The traditional electrolytes used in current batteries, which are like the liquid that carries charge back and forth, don’t work well with lithium metal.
They’re usually made up of salts dissolved in a liquid, and they don’t interact with lithium metal batteries the way we need them to.
To solve this, scientists have come up with a new mixture called localized high-concentration electrolytes.
These are crafted by mixing a lot of salt with a special liquid plus another ingredient called a diluent, which helps everything flow smoothly. This mixture is key because it lets electricity pass through the battery efficiently, which means more power and longer life.
The big breakthrough came when researchers understood that within these new electrolytes, there are tiny soap-like structures forming, similar to micelles.
In this microscopic world, the solvent (a part of the electrolyte mixture) acts like soap, connecting the salt and the diluent and wrapping them up.
This action, which had not been fully understood before, seems to be the reason why these new electrolytes are so promising for use in lithium metal batteries.
With this new understanding, the scientists can now figure out the best way to mix their ingredients. Getting the balance of salt, solvent, and diluent just right is crucial for making these new electrolytes work at their best.
The team at Idaho National Laboratory didn’t just stop at the theory. They put it into practice and, excitingly, found that it seems to hold up—they created batteries that keep going for longer. It’s like they found a recipe for battery success hidden within the workings of a bar of soap.
The researchers are thrilled by the potential of their discovery and are eager to see what new designs will emerge from their findings. But there’s still more work to do before they fully unlock the door to these super-powered batteries.
Professor Yue Qi from Brown’s School of Engineering summed it up nicely, expressing amazement at how a principle as familiar as soap’s cleaning mechanism could unlock such significant advances in battery technology.
Now, with a solid theory in hand, the team has a guide to creating the perfect electrolyte mix—a vital step toward the goal of batteries that could one day power a phone for a week or let an electric car drive for 500 miles without needing a charge.
Follow us on Twitter for more articles about this topic.



