In a significant development for the energy storage industry, researchers at the Center for Development of Functional Materials (CDMF) in Brazil have made promising strides in combating the issue of capacity loss in vanadium redox flow batteries.
These batteries, crucial in storing large amounts of energy for electric power utilities, have been the subject of an article published in the Chemical Engineering Journal.
Vanadium redox flow batteries are known for their ability to accumulate vast amounts of energy, making them ideal for industries like electric power utilities.
However, one of the challenges they face is capacity loss over time.
This happens due to a process known as transport loss, where ions leak between the anolyte (the positively charged component) and catholyte (the negatively charged component), leading to battery deactivation.
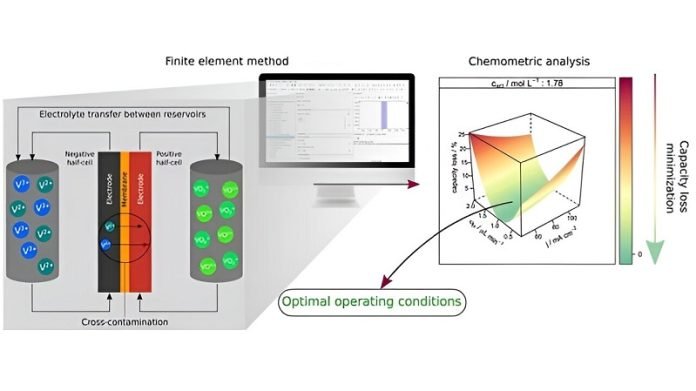
To tackle this issue, the Brazilian research team used computer simulations to understand how this ion leakage contributes to capacity loss and to find ways to mitigate it. Their goal was to keep the ion concentration constant over time, ensuring the battery maintains its charge capacity.
The researchers first estimated the impact of various factors like current density, concentration of active species (the substances that store and release energy), and the flow rate of these substances on the battery’s capacity loss.
Then, they looked for optimal conditions to minimize this loss. They focused on adjusting the flow between the electrolyte tanks to prevent cross-contamination, which is when these electroactive species unintentionally pass through the battery’s membrane.
Key Findings
The study revealed that current density and the concentration of active species are major factors influencing capacity loss. By adjusting the flow between electrolyte tanks, the researchers successfully mitigated the issue of cross-contamination under various operating conditions. This strategy proved effective in maintaining a stable ion concentration over time.
Ernesto Pereira, the senior author of the article and a professor at UFSCar, highlighted the main advantage of redox flow batteries: the lack of electrode aging.
Unlike other batteries where the electroactive components are coated onto electrodes, in redox flow batteries, these components are dissolved in solutions. This characteristic contributes to a longer battery life and minimal energy efficiency loss due to aging.
Looking Forward
The research, although conducted on a small scale, suggests that commercial vanadium redox flow batteries could potentially have a longer lifespan than other types.
The team is not only focusing on this aspect but also exploring the development of novel organic substances for use in these batteries, broadening the scope of their research.
In essence, this breakthrough by Brazilian researchers represents a significant step forward in enhancing the longevity and efficiency of vanadium redox flow batteries, paving the way for more sustainable and reliable energy storage solutions.