Usually, we think of water evaporating because of heat – like when sweat cools us down or morning dew disappears with the sunrise.
But scientists at MIT have discovered something really unexpected: under certain conditions, light can make water evaporate without any heat at all!
For a while now, some researchers were scratching their heads over an odd finding.
When they put water in a special sponge-like material called a hydrogel, it was evaporating way faster than it should, based only on the heat it was getting.
In some experiments, the water was vanishing at two or even three times the rate you’d expect.
A team at MIT decided to dig deeper. They ran new tests and looked back at other scientists’ work.
They ended up with a surprising answer: light, hitting the place where water meets air, can actually cause the water to evaporate. This happens more effectively than with heat!
The MIT researchers first looked into solar evaporation for cleaning saltwater, using a black material to absorb light and turn it into heat in the water.
Then they came across a study where a hydrogel made water evaporate double the maximum rate possible by heat alone.
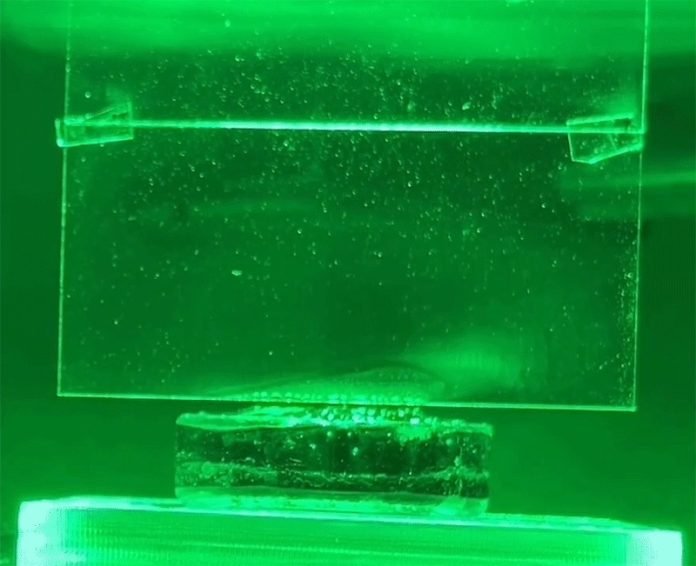
Skeptical at first, they tested a piece of the same hydrogel under simulated sunlight in their lab and, sure enough, the evaporation rate was off the charts.
The MIT team realized that light itself was likely nudging water molecules away from the surface. They experimented with different colors of light shining on a hydrogel filled with water.
They weighed the hydrogel to track how much water was lost and checked the temperature to ensure no extra heat was added.
They found that the evaporation rate changed with different light colors, peaking with a specific green light. This color effect is totally separate from heat, which confirmed that light alone was causing some of the evaporation.
Water and the hydrogel don’t typically absorb much light. But when combined, they can soak up solar energy really well. This lets them use the energy from light directly, bypassing the need for heat.
This discovery, which the MIT team calls the “photomolecular effect,” could be a game-changer.
It might help us understand and predict weather patterns like fog and cloud formation better. Plus, it could make solar-powered water cleaning much more efficient by skipping the step where sunlight is turned into heat first.
Right now, solar-powered methods can produce about 1.5 kilograms of clean water per square meter. But with this light-driven approach, we might increase that amount three or four times, making clean water cheaper and more accessible.
The researchers got funding to explore how this discovery can be used for better water cleaning and to study its impact on climate change.
They think it might also be helpful in other processes that need drying or cooling, using sunlight efficiently for cooling systems.
The team is working with others to replicate these surprising results and explore more applications. This finding could lead to new, cheaper ways to get fresh water and better understand our climate!
Follow us on Twitter for more articles about this topic.



