Picture a hot summer day, where the sun blazes in the sky and the air feels like it’s on fire.
This scene is becoming more frequent across our planet, with intense heatwaves and unexpected heavy rainfalls making our usual seasons somewhat topsy-turvy.
There’s a growing awareness that we need to take steps to ensure our planet can survive and continue to be a home for future generations.
One way we’re trying to do this is by using renewable energy – think solar panels on rooftops or giant wind turbines in fields.
However, these energy sources have their limitations, especially because weather (like sunny days or windy conditions) can be quite unpredictable.
This is where the concept of storing the generated energy comes in – using something called Energy Storage Systems (ESS).
These systems are a bit like giant batteries, holding onto the electricity generated on particularly sunny or windy days, to be used when the weather doesn’t cooperate.
Currently, many ESS use lithium-ion batteries (LIBs), which, while effective, come with their own set of challenges. They’re notably expensive and carry a risk of catching fire, which is less than ideal when we’re looking at widespread, safe usage.
A team of scientists led by Dr. Oh, Si Hyoung at the Korea Institute of Science and Technology (KIST) has taken a giant step towards creating a battery that is not only cheaper but also considerably safer.
Their research, published in the journal Energy Storage Materials, centers around aqueous rechargeable batteries.
The word ‘aqueous’ here simply means that the batteries use water-based solutions, making them significantly less costly due to the affordability of the raw materials compared to LIBs.
However, these aqueous batteries haven’t been without their own hurdles.
When they operate, hydrogen gas is unintentionally produced, gradually increasing the internal pressure of the battery and using up the electrolyte (the substance that helps produce electric charge). This situation, naturally, poses a safety risk and makes commercial use tricky.
In the past, researchers tried to work around this by adding a protective layer to minimize the contact between the metal anode (a battery component) and the electrolyte, but they were still stuck with inevitable corrosion and water decomposition issues. This continual buildup of hydrogen gas risked explosions during prolonged use.
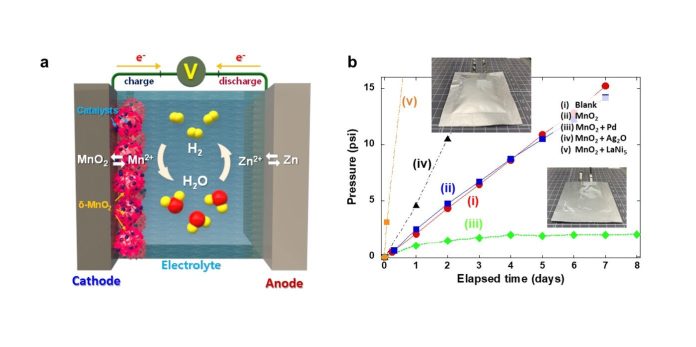
Addressing this, Dr. Oh’s team ingeniously developed a composite catalyst – think of it as a reactive agent – that consists of manganese dioxide and a pinch of palladium.
This catalyst can automatically convert the potentially problematic hydrogen gas back into water, maintaining the performance and safety of the battery.
Usually, manganese dioxide won’t react with hydrogen gas, but when paired with palladium, it does so readily, ensuring that any hydrogen produced is harmlessly transformed back into water.
In prototype testing, the internal pressure of batteries with this catalyst remained stable and safe, without losing electrolyte.
This innovative approach practically addresses a key safety concern for aqueous batteries and paves the way toward them being used in ESS in the future. If adopted widely, these cheaper and safer batteries could spur a growth spurt in the global ESS market.
Dr. Oh expressed that this technology is not only a custom safety strategy for batteries but also an automatic safety mechanism, controlling risks effectively.
He also envisaged its potential application in various industries, like hydrogen gas stations or nuclear power plants, where hydrogen gas leakage is a significant safety concern, safeguarding public safety in a broader sense.
In this imaginative leap towards managing our energy needs, researchers have given us a glimpse into a future where our energy might be stored safely and affordably, promoting a cleaner and more sustainable environment.
This promising development comes not a moment too soon as we grapple with the pressing need to power our world while caring for our shared home: Earth.
Follow us on Twitter for more articles about this topic.
Source: Korea Institute of Science and Technology.