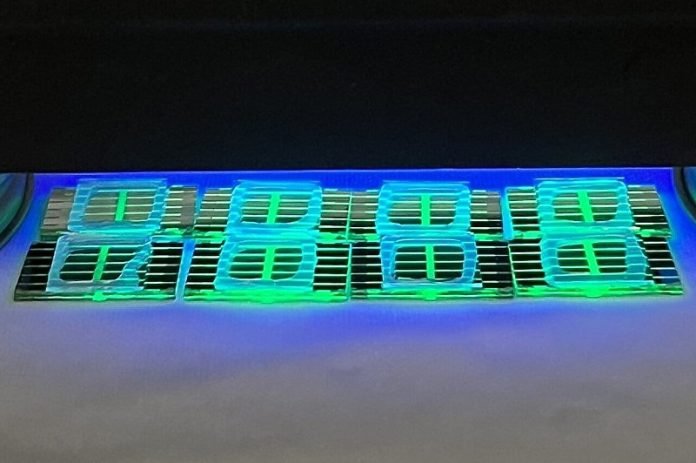
Light-emitting diodes (LEDs) are everywhere around us. They light up our homes, screens, and devices, from TVs to smartphones.
But, making LEDs can be tricky and costly. A team of researchers from Stanford University are trying to make a more efficient and affordable version using perovskite LEDs (PeLEDs).
Firstly, what are LEDs and how do they work? They’re tiny sources of light that work by passing an electric current through a type of crystal called a semiconductor.
However, these crystals are complicated to make and can be pretty expensive.
PeLEDs could be a game-changer. They use a different type of crystal made of a mix of various elements called metal halide perovskites.
The exciting thing is, these crystals can be made on glass, which is much cheaper than the materials used in regular LEDs.
It’s also possible to create PeLEDs by dissolving perovskites in a solution and painting it onto the glass. It’s like creating a bright layer of light with paint!
Not only could PeLEDs make LEDs more affordable, but they could also make colors on our screens look more vibrant. The green on your smartphone might look greener, and the blue, bluer.
However, there’s a catch. Currently, PeLEDs don’t last very long, and they’re less energy-efficient than regular LEDs. The reason?
Tiny gaps in the atomic structure of the perovskite crystals. These gaps are places where atoms should be, but aren’t. When energy enters these gaps, it doesn’t produce any light, so the overall efficiency of the PeLEDs decreases.
The Stanford team, led by Dan Congreve and Ph.D. student Sebastian Fernández, tried to fix this issue by replacing some of the lead atoms in the perovskites with manganese atoms.
This helped fill in the gaps, and it improved the PeLEDs’ brightness and efficiency. Their new-and-improved PeLEDs were more than twice as bright, nearly three times as efficient, and lasted about 37 minutes.
Fernández took the experiment a step further. He added an ingredient called TFPPO to the perovskite mix.
The result was a light that was up to five times more efficient and one of the brightest PeLEDs ever seen!
But, there was a downside. These super-bright lights lost half of their brightness in just two and a half minutes.
The team believes that the rapid fading of brightness is due to the TFPPO making it harder for electrical energy to become light. The TFPPO also seems to fill in some gaps in the atomic structure at first, but these gaps quickly reappear.
In the future, Fernández plans to try different ingredients to see if they can maintain the high efficiency of the PeLEDs without the lights fading so quickly. Meanwhile, the rest of the team is working on other challenges with PeLEDs, like making them produce bright violet and ultraviolet light.
If successful, these more affordable and efficient PeLEDs could be used to clean medical equipment, purify water, and even help grow plants indoors, offering a brighter future for everyone.
Follow us on Twitter for more articles about this topic.
Source: Stanford University.



