We’ve all heard about how our planet is getting warmer because of the greenhouse gases we’ve been releasing for the past few centuries.
A lot of these harmful gases come from burning fossil fuels like coal and oil, especially since the start of the Industrial Revolution.
With more cars, factories, and power plants, we’ve been releasing a ton of carbon dioxide (CO2) into the air.
To fix this problem, countries like the United States have made a promise.
By 2030, they want to cut down the amount of greenhouse gases they release by more than half compared to 2005. This is part of a worldwide plan to have zero extra greenhouse gases by 2050.
But, how do we achieve this? Especially when power plants and industries release so much CO2? Well, some smart researchers might have a solution.
Recently, scientists from different universities teamed up to find a new way to use this CO2. They published their findings in a journal called Nature Energy.
Their idea? Convert the CO2 we release into propane, which is a type of fuel that’s cleaner and stores more energy.
Andrew Rappe, one of the researchers, explained that this method could be a way to store energy from renewable sources like wind or solar.
It also helps in reducing the CO2 in the air. Another researcher, Mohammad Asadi, mentioned that this technique could allow us to keep using many products we’re used to but in a cleaner way.
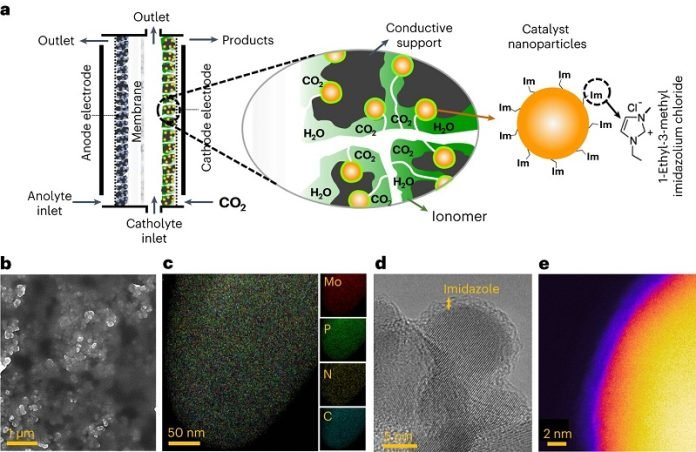
In the past, scientists tried to turn CO2 into other fuels. They mainly used copper for this process. The problem was, they could only make fuels like methane, which doesn’t store as much energy. Propane, which they can now produce, has much more energy.
The tricky part was figuring out how to get propane instead of methane. Zhen Jiang, who was a major part of the study, said that the usual methods didn’t work very well for propane. They needed a fresh approach.
That’s when they thought of adding something called an ionic liquid (IL) to the process. They also used a different material, called tri-molybdenum phosphide (Mo3P), instead of copper. When they combined these in their experiments, they found a big win: they could turn CO2 into propane with an amazing 91% efficiency!
This discovery also gave the scientists a new way to look at how different materials can help in these processes. They realized that by using techniques like coating with IL, they could improve the results even more.
So, what’s next? The researchers have two main goals. First, they want to study more about these ionic liquids to see which ones work best. Second, they want to find other materials that can turn CO2 into even better fuels. They dream of making everything from gas to jet fuel from the CO2 we produce.
In the end, Rappe summed up their hopes nicely. He said that by using this research, we could keep reusing the same carbon atoms for energy without releasing them into the air. This way, we get the power we need, and our planet stays cleaner and cooler. A win-win for everyone!
Follow us on Twitter for more articles about this topic.
Source: University of Pennsylvania.



