You’ve probably heard of lithium-ion batteries. They’re everywhere, from your phone to electric cars.
But what if we could make batteries using something even more common than lithium, like the stuff found in seawater?
Researchers at Worcester Polytechnic Institute (WPI) have discovered a new way to make batteries using chloride ions, which are plentiful in seawater.
This exciting discovery was made by Xiaowei Teng, a top expert in Chemical Engineering at WPI.
Here’s why this is big news: Lithium-ion batteries, though powerful, have some issues. They’re expensive and rely on materials like cobalt, nickel, and of course, lithium.
These materials aren’t found everywhere. In fact, just six countries have most of the world’s lithium. That makes it hard and costly to get.
But what if we could make batteries using materials that are easier to find and better for our planet? That’s where Teng and his team come in. Alongside researchers from the University of Alabama and Brookhaven National Laboratory in New York, they figured out how to use chloride ions from seawater in batteries.
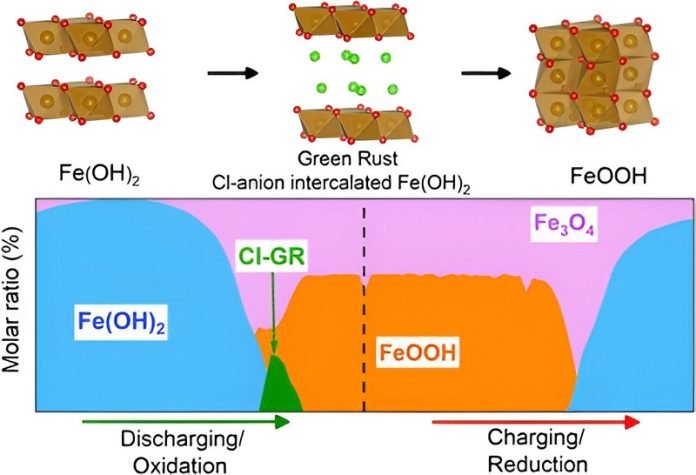
Their research was shared in a paper titled “Chloride-Insertion Enhances the Electrochemical Oxidation of Iron Hydroxide Double Layer Hydroxide into Oxyhydroxide in Alkaline Iron Batteries”, which can be found in the Chemistry of Materials journal.
So, what did they find out? They noticed that when chloride ions mixed with a particular kind of iron compound, it created a material that helped batteries hold and release energy. The best part? This process was more stable and could be used many times over.
But they didn’t stop there. To make sure their discovery was solid, Teng and his student, Sathya Narayanan Jagadeesan, took their findings to the Department of Energy User Facilities at Brookhaven National Laboratory. There, they ran special tests to confirm that their new battery worked as they thought.
Finally, the WPI researchers made a prototype battery using their new method. This battery used materials that are easy to find, especially iron, which exists in large quantities.
Teng believes that this new battery could be cheaper because it uses materials that are common and sometimes even discarded. For instance, in the U.S., a lot of iron waste isn’t recycled, and much of it just rusts away. With this new battery technique, we might be able to turn that rust into a source of power.
In simple terms, this means that in the future, our batteries could be made using materials from the sea and recycled waste. That’s not only good for our wallets but also great for the environment.
In conclusion, this research from WPI offers a promising look into the future of batteries. It’s a future where our energy storage might just come from the sea, and where waste could be turned into power.
The next time you charge your phone or drive an electric car, remember that the energy might one day come from a greener and more sustainable battery, thanks to innovations like this.
Follow us on Twitter for more articles about this topic.
Source: Worcester Polytechnic Institute.



