Scientists at the University of Michigan have discovered that cracking in lithium-ion batteries’ positive electrodes, a problem that was perceived as detrimental, can actually help decrease charging time.
This goes against the conventional approach of electric vehicle manufacturers, who have been trying to reduce cracking, believing that it reduces the life of the batteries.
Professor Yiyang Li, a materials science and engineering expert, stated, “Companies are keen on creating ‘million-mile’ batteries that don’t crack.
But removing these cracks would mean less surface area for charging, leading to longer charging times.”
The Role of Cathode Particles in Battery Charging
The team’s findings are relevant for more than half of the electric vehicle batteries in use.
In these batteries, the positive electrode, also known as the cathode, is made up of trillions of tiny particles composed of lithium nickel manganese cobalt oxide or lithium nickel cobalt aluminum oxide.
Typically, charging speed is determined by the cathode particles’ surface-to-volume ratio. The smaller the particle, the faster it should charge.
This is because a smaller particle has a higher surface area in proportion to its volume, meaning the lithium ions have less distance to cover as they move through it.
Until now, the accepted understanding about the charging speed and particle size has been based more on assumptions than concrete data, because conventional methods only measured the charging speed on average, not individual cathode particles.
Cracks in Cathode Particles Increase Active Surfaces
The team found that cracked cathode particles have more active surfaces that can accommodate lithium ions, both on their outer surface and within the cracks.
Jinhong Min, a PhD student in Professor Li’s lab, said, “Battery scientists are aware that cracking happens, but no one has measured how it affects the charging speed before.”
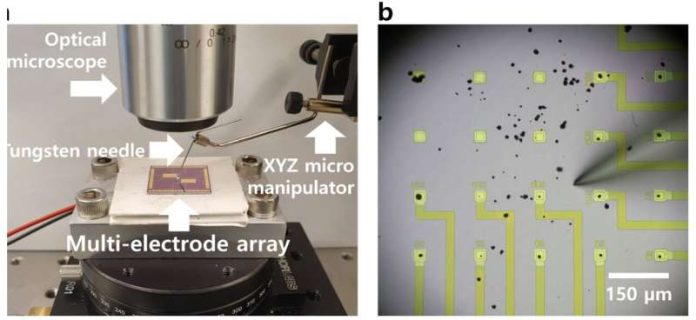
The scientists accomplished the task of measuring the charging speed of individual particles by using a device typically used by neuroscientists to study electrical signals in individual brain cells.
Each array in the device, a small chip with multiple microelectrodes, allowed the researchers to charge and discharge individual particles.
Their experiments showed that the charging speed did not depend on particle size. The researchers believe that when larger particles crack, they behave like a group of smaller particles.
Another possibility is that the lithium ions move quickly within the tiny spaces between the nanoscale crystals that make up the cathode particle.
Conclusions and Future Directions
The results of the study have implications for the design of long-lasting batteries. Single-crystal particles that do not crack may need to be smaller than current cracking cathode particles to charge quickly.
Alternatively, different materials could be used to create single-crystal cathodes, but these materials may face limitations such as metal supply constraints or lower energy densities.
Li concludes, “Cracks are important to consider when designing batteries for future electric vehicles.”
The study was published in Energy & Environmental Science.