How would you like a world powered by clean and efficient energy sources instead of fossil fuels?
Well, that might not be as far away as it seems.
A group of scientists at the RIKEN Center for Emergent Matter Science (CEMS) in Japan have found a way to store hydrogen safely, which is an essential step towards a cleaner, more sustainable energy future.
Hydrogen is a powerful source of energy but has a catch – it’s highly explosive, so storing and transporting it safely is a big challenge.
A workaround solution is to pack hydrogen into another molecule and extract it when needed.
Ammonia, a compound made up of one nitrogen atom and three hydrogen atoms (NH3), is a great choice for this since about 20% of its weight comes from hydrogen.
The trouble is, ammonia is a corrosive gas, and dealing with it can be quite tricky. It’s usually stored in a liquid state at super-cold temperatures in strong, pressure-resistant containers.
Another method is to store it in porous compounds at room temperature, but the storage capacity is low, and it’s not always easy to get the ammonia back out.
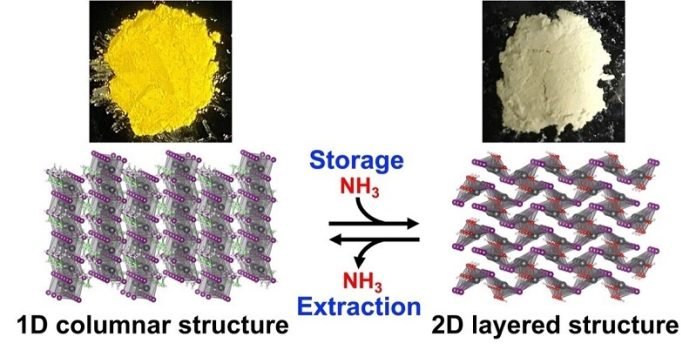
This is where the RIKEN team’s discovery comes in. They found a special type of crystal material known as a perovskite that can absorb and hold ammonia conveniently and allow easy extraction at low temperatures.
The perovskite they focused on is called ethylammonium lead iodide (EAPbI3). When EAPbI3 reacts with ammonia at normal room temperature and pressure, it changes into a layered structure known as lead iodide hydroxide, or Pb(OH)I.
This transformation allows the perovskite to store the ammonia safely, and it’s cheaper and simpler than current methods of storing ammonia.
The best part? Extracting the stored ammonia is a piece of cake! According to Masuki Kawamoto, who led the research, the stored ammonia can be easily recovered by gently heating the material.
The compound goes through a reverse reaction at 50°C (122°F), much lower than the 150°C (302°F) required with porous compounds.
An added bonus is that the perovskite changes color when it stores ammonia, going from yellow to white. This could lead to the development of color-based sensors to monitor how much ammonia is stored.
Not only does this research make storing ammonia, an essential compound used in fertilizers, pharmaceuticals, and textiles, safer, but it also brings us one step closer to a cleaner energy future.
As per Yoshihiro Ito, a co-author of the study, this could lead to a ‘decarbonized society’ where hydrogen carried by ammonia replaces carbon-based energy.
So, the discovery at RIKEN is another leap towards reaching the United Nations’ Sustainable Development Goals for 2016.
They aim for affordable, clean energy (Goal 7) and decisive action against climate change (Goal 13). It looks like a future full of clean energy is no longer just a dream, but a real possibility!
Follow us on Twitter for more articles about this topic.



