What if we could turn sunlight into a sustainable and clean fuel source? Sounds like science fiction, right?
But it’s actually what a team of scientists led by Professor Dominik Eder from the Institute of Materials Chemistry at TU Wien is working on.
They’re exploring ways to produce hydrogen, a green fuel, using sunlight and a special ingredient called a catalyst.
Their exciting research was recently published in the journal Advanced Energy Materials.
Usually, hydrogen production is not very eco-friendly because it emits a lot of carbon dioxide (CO2), a gas that contributes to climate change.
That’s where photocatalysis comes in. This process can split water molecules into hydrogen and oxygen using light and a catalyst. So, we could literally store the sun’s energy in hydrogen fuel.
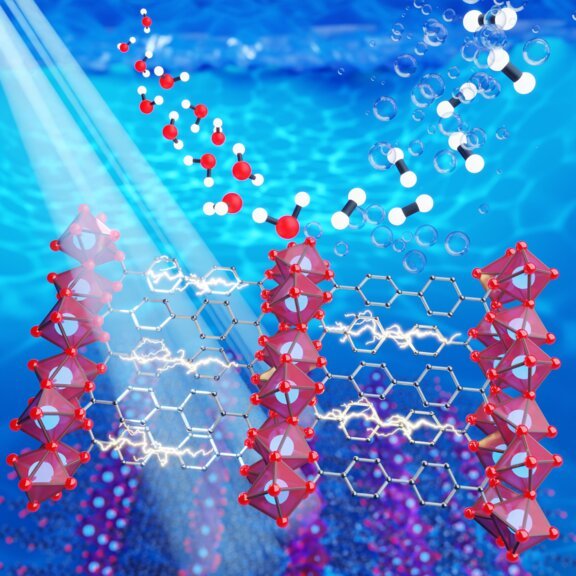
Now, let’s talk about the catalyst. It’s like the secret sauce in this process. The catalyst being used by Prof. Eder’s team is a type of substance known as a metal-organic framework, or MOF. MOFs are compounds made up of metal ions and organic molecules.
Imagine them as really tiny 3D networks that are great at absorbing light and splitting charges, which is essential for photocatalysis.
But here’s the catch. Most MOFs work well with UV light, but not so much with visible light, which is a big chunk of sunlight. Plus, modifying these MOFs to absorb visible light could mess with how the electrons move around, which isn’t good.
To tackle this issue, the team looked at layered MOFs. Pablo Ayala, the study’s lead author, compares them to a wafer bar, with the wafer being the inorganic part and the chocolate acting as the glue holding everything together.
The challenge, though, is that these layered MOFs are usually non-porous, meaning the surface area for the reaction is quite limited. The team needed to make these particles super small while ensuring they were still perfect.
Luckily, Prof. Eder’s team developed a new way to create these tiny structures without any defects.
The new MOFs are cube-shaped and only a few nanometers in size, about 1000 times thinner than a strand of hair! The best part? These MOFs broke records in producing hydrogen when exposed to visible light.
The team used computer simulations to understand how the reaction works. They discovered that the layered structure of the MOFs was key to efficiently separating and extracting charges. They also found out that any missing parts in the MOF acted as traps for charges, which could affect how well the photocatalysis worked.
So, the next step for these scientists is to design new types of layered MOFs and explore their potential for energy applications.
Who knows, maybe one day our cars or houses could be powered by the sun’s energy stored in hydrogen fuel, all thanks to these tiny, cleverly designed catalysts!
Follow us on Twitter for more articles about this topic.
Source: Vienna University of Technology.