Teams of scientists from Flinders University in Australia and Zhejiang Sci-Tech University in China are making strides toward creating the world’s first safe, efficient, and eco-friendly aluminum battery!
This significant development was outlined in a report titled, “Lewis Acid-Induced Reversible Disproportionation of TEMPO Enables Aqueous Aluminum Radical Batteries,” which was published by the Journal of the American Chemical Society.
Present-day batteries are kind of a double-edged sword. On one hand, they power our devices, which is great, right?
But on the other hand, they’re filled with hazardous stuff that’s harmful to the environment.
Materials like lead, cadmium, and mercury that are found in most batteries can pollute the environment and even poison people and animals. What’s worse is they hang around in our soil and water for a long time after the batteries are thrown away.
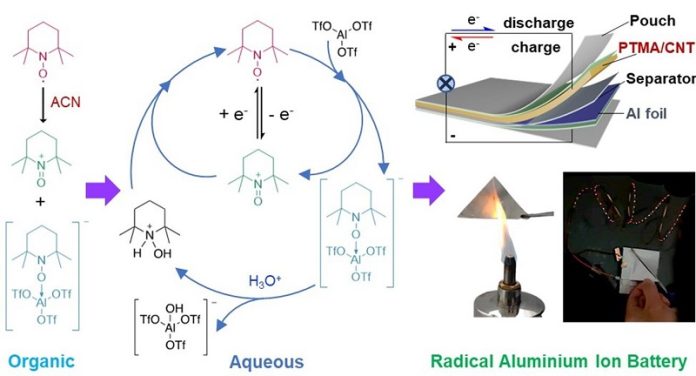
Now, here’s where our scientists come in. Dr. Kai Zhang from China and Professor Zhongfan Jia’s team from Australia worked together on a project that involves something called stable radicals, which play a vital role in the performance of batteries.
They have come up with a new design for aluminum batteries.
These batteries use water-based electrolytes, meaning they are safer, more stable, and don’t easily catch fire. The initial tests showed promising results: these batteries can work effectively for over 800 cycles with only a minimal loss of efficiency.
And there’s more! Professor Jia hopes that, in the future, these batteries can be made using biodegradable materials. This would make these new batteries not just safe to use, but also eco-friendly.
Now, here’s a cool fact: batteries using aluminum ions can hold more energy than the lithium-ion batteries we commonly use in our devices. Why is that? Well, it’s because they use elements like aluminum, zinc, or magnesium that are abundantly available on our planet.
But the problem is, in current aluminum-ion batteries, the aluminum ions move pretty slowly, which affects their performance.
The use of something called organic conjugated polymers was supposed to solve this problem, but so far, their results weren’t very impressive.
Here’s where the “stable radicals” come back into play. These are unique organic molecules that can be used in batteries. The first battery of this kind was made commercially available by NEC in 2012.
The research lab at Flinders University has already used these radicals in various other batteries. But their use in aluminum-ion batteries is brand new, mainly because we didn’t fully understand how they would react.
This new research is a big step forward in creating batteries that are not just efficient and safe, but also environmentally friendly. It’s like having our cake and eating it too!
Follow us on Twitter for more articles about this topic.



