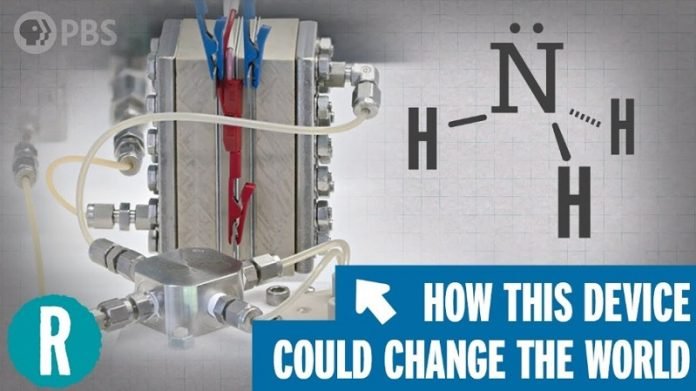
The Haber-Bosch process is like a super important recipe that helped feed the world!
Two smart guys, Fritz Haber, and Carl Bosch, came up with it about a hundred years ago.
They figured out how to take nitrogen from the air and hydrogen and turn it into ammonia.
This might not sound that exciting, but ammonia is used to make fertilizer which helps plants grow better.
Think about it like this: nitrogen is like food for plants, but the nitrogen in the air is locked away in a form plants can’t use.
The Haber-Bosch process is like a key that unlocks the nitrogen, making it into a form plants can eat!
But there are some problems. The process needs a lot of energy (like 1% of all the energy we use in the world!) and it also produces a lot of CO2, a gas that’s causing climate change.
So, for over a hundred years, scientists have been trying to find a better way to do this, one that doesn’t use so much energy or make as much CO2.
Chemists have been trying to come up with a fundamentally better way to fix nitrogen for over a century.
Have they finally succeeded?
Reactions is a video series produced by the American Chemical Society and PBS Digital Studios.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress.
ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people.
The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News.
ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
Follow us on Twitter for more articles about this topic.



