Everyone is talking about lithium-ion batteries these days, and you can see why.
They’re everywhere! In your smartphone, in your laptop, even in electric cars.
But there’s a problem: lithium and other materials we need to make these batteries aren’t found everywhere in the world.
That means we need to rely on certain countries to supply these materials, and it can be tough to guarantee a stable supply chain.
What if we could use a material that’s not just more common, but also has the potential to make batteries with even more energy?
That’s the question Dr. Minah Lee and her team at the Korea Advanced Institute of Science and Technology (KIST) have been tackling. They’re looking at magnesium, a metal that’s way more common on Earth and could potentially lead to high-capacity batteries.
Here’s the thing: magnesium has twice the charge of lithium (it’s what we call a divalent ion), meaning that if used correctly, we could potentially store a lot more energy in the same size battery.
Magnesium metal, if used as the anode (the battery’s negative terminal), has around 1.9 times higher capacity than lithium metal. That’s a lot of extra power!
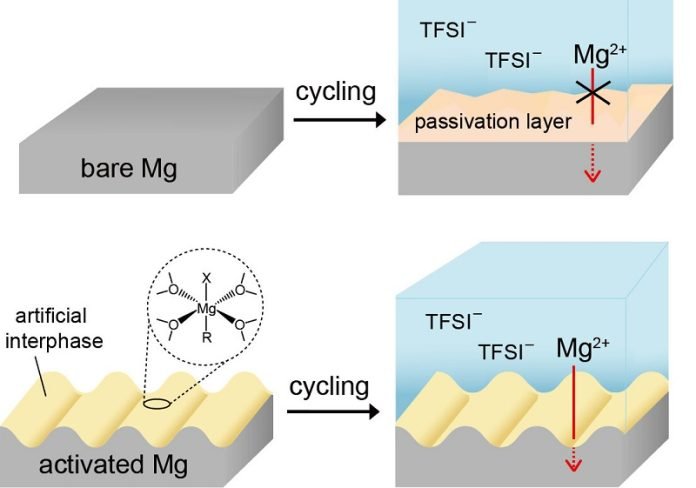
The problem, though, has been that magnesium is super reactive with electrolytes – the stuff that lets the electric charge flow in a battery.
That’s made it tricky to use magnesium in batteries effectively. However, the KIST team has cracked the code, finding a way to get magnesium to charge and discharge efficiently.
Instead of using corrosive electrolytes that can damage the battery, Dr. Lee’s team used a common electrolyte much like the ones in existing batteries. This reduces damage and allows for high-voltage electrodes.
The team also came up with a smart way to protect the magnesium. They created an artificial protective layer made of magnesium alkyl halide oligomers by simply dipping the magnesium anode into a reactive solution before putting the battery together.
This helped create nanostructures on the magnesium surface that improved the charging and discharging process.
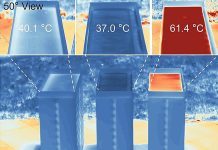
The new approach dropped the overpotential – the excess energy required to make a reaction happen – from more than 2 volts to less than 0.2 volts. The battery’s efficiency jumped from less than 10% to more than 99.5%.
And the best part? This new magnesium battery was able to recharge and discharge steadily for more than 990 cycles!
Dr. Lee hopes this research will open up a new direction in the field. With this new method, we could see the development of cheaper, higher-capacity batteries that are perfect for storing large amounts of energy.
Imagine the possibilities for our phones, cars, and even for renewable energy storage systems! It seems like magnesium might just be the superhero in the story of future battery technology.
The study was published in the journal ACS Nano.
Source: Korea Institute of Science and Technology.