A group of scientists led by Professor Armido Studer at the Institute of Organic Chemistry at Münster University in Germany, has come up with an innovative way to split water molecules into hydrogen gas, a process known as water splitting.
This method could potentially pave the way for cleaner energy production, and aid in the manufacture of various crucial substances, including pharmaceutical drugs.
Water splitting is a process where water (H2O) is broken down into its basic components – hydrogen gas (H2) and oxygen (O2).
However, it’s not as easy as it sounds because water molecules are extremely stable and don’t like to be separated.
To get around this, scientists have to use a catalyst, a substance that speeds up a chemical reaction without itself getting consumed.
Usually, this catalyst is made from transition metals, elements in the middle of the periodic table that can interact with other elements in interesting ways.
But Prof. Studer’s team decided to try a new approach.
They used triaryl phosphines, a type of organic (carbon-based) molecule, instead of metal complexes.
Even more exciting, they harnessed the power of light to drive the reaction – hence, it’s called a photocatalytic process.
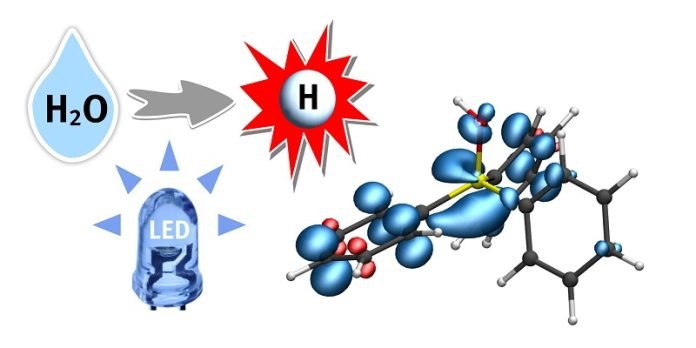
Here’s where the real chemistry magic happens. The phosphine interacts with the water, creating an intermediate substance, a “phosphine-water radical cation.”
In simple terms, this substance is like activated water, ready to have its hydrogen atoms plucked off and used elsewhere.
Dr. Christian Mück-Lichtenfeld, who examined this activated water, explains that the bond between hydrogen and oxygen in this intermediate substance is very weak. This allows a hydrogen atom to be easily transferred to other compounds, which opens up a whole new world of possibilities!
One key application of this discovery is in hydrogenation reactions, explains Dr. Jingjing Zhang, who conducted the experiments. In hydrogenation, hydrogen is added to other molecules, like alkenes and arenes. This process is used a lot in creating medicines, agrochemicals, and materials.
This new method of water splitting using light energy and phosphines is a breakthrough, and it could provide a new platform to explore uncharted chemical processes.
Moreover, the process could help develop new methods of hydrogen production, which is an environmentally friendly energy source of the future.
The study was published in Nature.