Scientists have made a remarkable discovery that could help combat climate change and revolutionize chemical production.
They have developed a special material that can efficiently convert methane, a potent greenhouse gas, into formaldehyde, a versatile and essential chemical.
This breakthrough could pave the way for more eco-friendly and waste-free manufacturing processes. Let’s explore this exciting research and its potential implications.
The Challenge of Methane Conversion: Methane, a major component of natural gas, is not only widely used but also a significant contributor to global warming.
It possesses more than 70 times the global warming potential of carbon dioxide. Scientists have been exploring ways to convert methane into other chemicals as a means to achieve a sustainable energy and chemical supply while addressing environmental concerns.
However, methane is a remarkably stable molecule, making it challenging to convert it into useful products.
The Power of Formaldehyde: Formaldehyde is a chemical of great importance, with a market value of USD 8 billion and various applications in everyday life.
It serves as a crucial component in household, commercial, medical, and automotive products.
Moreover, it is used in the production of essential materials such as melamine, urea-formaldehyde, and phenolic resins.
Currently, formaldehyde is typically produced through energy-intensive processes that result in substantial carbon dioxide emissions.
Harnessing Sunlight for Conversion: The research team discovered an innovative approach to convert methane into formaldehyde using sunlight.
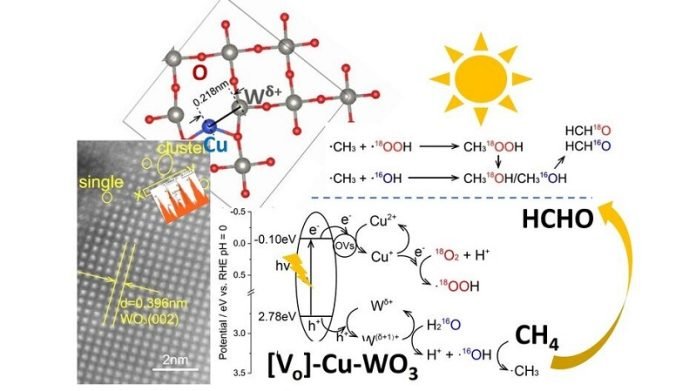
They developed a special material derived from tungsten trioxide (WO3 catalyst), which contains copper and tungsten atomic species. These two elements work together to ensure an effective and selective conversion process.
The researchers found that under visible light, their material achieved nearly 100% selectivity, meaning it produced very little unwanted byproducts.
Understanding the Process: Through in-depth analysis, the scientists uncovered how their catalyst works. The copper atoms in the material help move electrons and create reactive molecular species, while the tungsten atoms activate the methane gas.
The copper acts as electron acceptors, facilitating the transfer of electrons and generating reactive hydroperoxyl radicals. Simultaneously, the adjacent tungsten atoms with a partial positive charge act as hole acceptors.
Water molecules present in the process produce hydroxyl radicals, which effectively activate methane, leading to the formation of valuable formaldehyde.
The collaboration between copper and tungsten sites greatly enhances the overall efficiency and selectivity of the conversion process.
Implications and Future Prospects: This groundbreaking research opens up new possibilities for developing more sustainable and efficient chemical conversion processes.
By harnessing sunlight, methane, a harmful greenhouse gas, can be transformed into valuable chemicals such as formaldehyde.
The findings have significant implications for reducing carbon dioxide emissions and energy consumption associated with traditional production methods. Furthermore, this discovery may pave the way for the development of new catalysts for various chemical conversions, promoting a greener future for the chemical industry.
The collaboration between scientists from different universities has led to a remarkable breakthrough in converting methane into formaldehyde using sunlight.
This innovative process offers a potential solution to both environmental concerns and the growing demand for valuable chemicals.
By utilizing a specially designed catalyst, the researchers have achieved high selectivity and efficiency, outperforming previous methods. The implications of this research extend beyond methane conversion, paving the way for more sustainable and efficient chemical processes.
With further exploration and development, this breakthrough could have a profound impact on combating climate change and transforming the chemical industry towards a greener future.
Source: University of Hong Kong.



