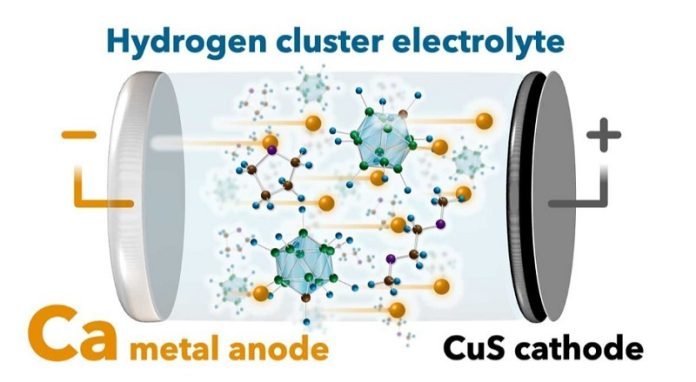
Scientists have developed a prototype calcium (Ca) metal rechargeable battery capable of 500 cycles of repeated charge-discharge – the benchmark for practical use.
With the use of electric vehicles and grid-scale energy storage systems on the rise, the need to explore alternatives to lithium-ion batteries (LIBs) has never been greater. One such replacement is Ca metal batteries.
As the fifth most abundant element in earth’s crust, calcium is widely available and inexpensive and has higher energy density potential than LIBs.
Its properties are also thought to help accelerate ion transport and diffusion in electrolytes and cathode materials, giving it an edge over other LIB-alternatives such as magnesium and zinc.
But many hurdles remain in the way of Ca metal batteries’ commercial viability. The lack of an efficient electrolyte and the absence of cathode materials with sufficient Ca2+ storage capabilities have proved to be the main stumbling blocks.
Back in 2021, some members of the current research group provided a solution to the former problem when they realized a new fluorine-free calcium (Ca) electrolyte based on a hydrogen (monocarborane) cluster.
The electrolyte demonstrated markedly improved electrochemical performances such as high conductivity and high electrochemical stabilities.
“For our current research, we tested the long-term operation of a Ca metal battery with a copper sulfide (CuS) nanoparticle/carbon composite cathode and a hydride-based electrolyte,” says Kazuaki Kisu, assistant professor at Tohoku University’s Institute for Materials Research (IMR).
Also a natural mineral, CuS has favorable electrochemical properties. Its layered structure enables it to store a variety of cations, including lithium, sodium and magnesium. It has a large theoretical capacity of 560 mAh g-1 – two to three times higher than present cathode materials for lithium-ion batteries.
Through nanoparticulation and compositing with carbon materials, Kisu, and his colleagues were able to create a cathode capable of storing large amounts of calcium ions.
When employed with the hydride-type electrolyte, they produce a battery with highly stable cycling performance. The prototype battery maintained 92% capacity retention over 500 cycles based on the capacity of the 10th cycle.
The group is confident that their breakthrough will help advance research into cathode materials for Ca-based batteries.
“Our study confirms the feasibility of Ca metal anodes for long-term operations, and we are hopeful the results will expedite the development of Ca metal batteries,” says Kisu.
The breakthrough was reported in the journal Advanced Science.



