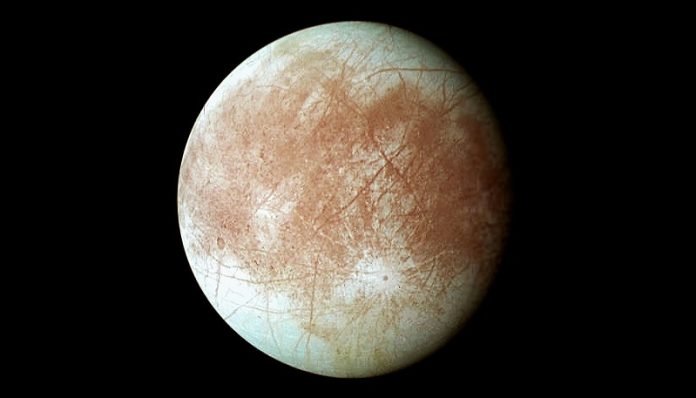
A new substance created in a lab on Earth could form at the surface and bottom of Europa’s deep oceans, say researchers.
The red streaks crossing the surface of Jupiter’s moon are thought to be a frozen mixture of water and salts, but its chemical signature matches no known substance on Earth.
The researchers may have solved the puzzle with the discovery of a new type of solid crystal that forms when water and table salt combine in cold and high-pressure conditions.
The study, published in the Proceedings of the National Academy of Sciences, announces a new combination for two of Earth’s most common substances: water and sodium chloride, or table salt.
“It’s rare nowadays to have fundamental discoveries in science,” says lead author Baptiste Journaux, an acting assistant professor of earth and space sciences at the University of Washington.
“Salt and water are very well known at Earth conditions. But beyond that, we’re totally in the dark. And now we have these planetary objects that probably have compounds that are very familiar to us, but at very exotic conditions. We have to redo all the fundamental mineralogical science that people did in the 1800s, but at high pressure and low temperature. It is an exciting time.”
At cold temperatures, water and salts combine to form a rigid salted icy lattice, known as a hydrate, held in place by hydrogen bonds. The only previously known hydrate for sodium chloride was a simple structure with one salt molecule for every two water molecules.
But the two new hydrates, found at moderate pressures and low temperatures, are strikingly different. One has two sodium chlorides for every 17 water molecules; the other has one sodium chloride for every 13 water molecules. This would explain why the signatures from the surface of Jupiter’s moons are more “watery” than expected.
“It has the structure that planetary scientists have been waiting for,” Journaux says.
The discovery of new types of salty ice has importance not just for planetary science, but for physical chemistry and even energy research, which uses hydrates for energy storage, Journaux says.
The experiment involved compressing a tiny bit of salty water at synchrotron facilities in France, Germany and the US between two diamonds about the size of a grain of sand, squeezing the liquid up to 25,000 times the standard atmospheric pressure. The transparent diamonds allowed the team to watch the process through a microscope.
“We were trying to measure how adding salt would change the amount of ice we could get, since salt acts as an antifreeze,” Baptiste says. “Surprisingly, when we put the pressure on, what we saw is that these crystals that we were not expecting started growing. It was a very serendipitous discovery.”
Such cold, high-pressure conditions created in the lab would be common on Jupiter’s moons, where scientists think 5 to 10 kilometers (3 to 6 miles) of ice would cover oceans up to several hundred kilometers thick, with even denser forms of ice possible at the bottom.
“Pressure just gets the molecules closer together, so their interaction changes—that is the main engine for diversity in the crystal structures we found,” Journaux says.
Once the newly discovered hydrates had formed, one of the two structures remained stable even after the pressure was released.
“We determined that it remains stable at standard pressure up to about minus 50 C. So if you have a very briny lake, for example in Antarctica, that could be exposed to these temperatures, this newly discovered hydrate could be present there,” Journaux says.
The team hopes to either make or collect a larger sample to allow more thorough analysis and verify whether the signatures from icy moons match the signatures from the newly discovered hydrates.
Two upcoming missions will explore Jupiter’s icy moons: The European Space Agency’s Jupiter Icy Moons Explorer mission, launching in April, and NASA’s Europa Clipper mission, launching for October 2024. NASA’s Dragonfly mission launches to Saturn’s moon Titan in 2026. Knowing what chemicals these missions will encounter will help to better target their search for signatures of life.
“These are the only planetary bodies, other than Earth, where liquid water is stable at geological timescales, which is crucial for the emergence and development of life,” Journaux says.
“They are, in my opinion, the best place in our solar system to discover extraterrestrial life, so we need to study their exotic oceans and interiors to better understand how they formed, evolved and can retain liquid water in cold regions of the solar system, so far away from the sun.”
Written by Hannah Hickey



