Scientists have found an effective new method for removing carbon dioxide from the ocean.
As carbon dioxide continues to build up in the Earth’s atmosphere, research teams around the world have spent years seeking ways to remove the gas efficiently from the air.
Meanwhile, the world’s number one “sink” for carbon dioxide from the atmosphere is the ocean, which soaks up some 30 to 40 percent of all of the gas produced by human activities.
Recently, the possibility of removing carbon dioxide directly from ocean water has emerged as another promising possibility for mitigating CO2 emissions, one that could potentially someday even lead to overall net negative emissions.
But, like air capture systems, the idea has not yet led to any widespread use, though there are a few companies attempting to enter this area.
Now, a team of researchers at MIT says they may have found the key to a truly efficient and inexpensive removal mechanism.
The findings were reported this week in the journal Energy and Environmental Science, in a paper by MIT professors T. Alan Hatton and Kripa Varanasi, postdoc Seoni Kim, and graduate students Michael Nitzsche, Simon Rufer, and Jack Lake.
The existing methods for removing carbon dioxide from seawater apply a voltage across a stack of membranes to acidify a feed stream by water splitting.
This converts bicarbonates in the water to molecules of CO2, which can then be removed under vacuum.
Hatton, who is the Ralph Landau Professor of Chemical Engineering, notes that the membranes are expensive, and chemicals are required to drive the overall electrode reactions at either end of the stack, adding further to the expense and complexity of the processes.
“We wanted to avoid the need for introducing chemicals to the anode and cathode half cells and to avoid the use of membranes if at all possible,” he says.
The team came up with a reversible process consisting of membrane-free electrochemical cells. Reactive electrodes are used to release protons to the seawater fed to the cells, driving the release of the dissolved carbon dioxide from the water.
The process is cyclic: It first acidifies the water to convert dissolved inorganic bicarbonates to molecular carbon dioxide, which is collected as a gas under vacuum.
Then, the water is fed to a second set of cells with a reversed voltage, to recover the protons and turn the acidic water back to alkaline before releasing it back to the sea.
Periodically, the roles of the two cells are reversed once one set of electrodes is depleted of protons (during acidification) and the other has been regenerated during alkalization.
This removal of carbon dioxide and reinjection of alkaline water could slowly start to reverse, at least locally, the acidification of the oceans that has been caused by carbon dioxide buildup, which in turn has threatened coral reefs and shellfish, says Varanasi, a professor of mechanical engineering.
The reinjection of alkaline water could be done through dispersed outlets or far offshore to avoid a local spike of alkalinity that could disrupt ecosystems, they say.
“We’re not going to be able to treat the entire planet’s emissions,” Varanasi says. But the reinjection might be done in some cases in places such as fish farms, which tend to acidify the water, so this could be a way of helping to counter that effect.
Once the carbon dioxide is removed from the water, it still needs to be disposed of, as with other carbon removal processes.
For example, it can be buried in deep geologic formations under the sea floor, or it can be chemically converted into a compound like ethanol, which can be used as a transportation fuel, or into other specialty chemicals.
“You can certainly consider using the captured CO2 as a feedstock for chemicals or materials production, but you’re not going to be able to use all of it as a feedstock,” says Hatton. “You’ll run out of markets for all the products you produce, so no matter what, a significant amount of the captured CO2 will need to be buried underground.”
Initially at least, the idea would be to couple such systems with existing or planned infrastructure that already processes seawater, such as desalination plants.
“This system is scalable so that we could integrate it potentially into existing processes that are already processing ocean water or in contact with ocean water,” Varanasi says.
There, the carbon dioxide removal could be a simple add-on to existing processes, which already return vast amounts of water to the sea, and it would not require consumables like chemical additives or membranes.
“With desalination plants, you’re already pumping all the water, so why not co-locate there?” Varanasi says. “A bunch of capital costs associated with the way you move the water, and the permitting, all that could already be taken care of.”
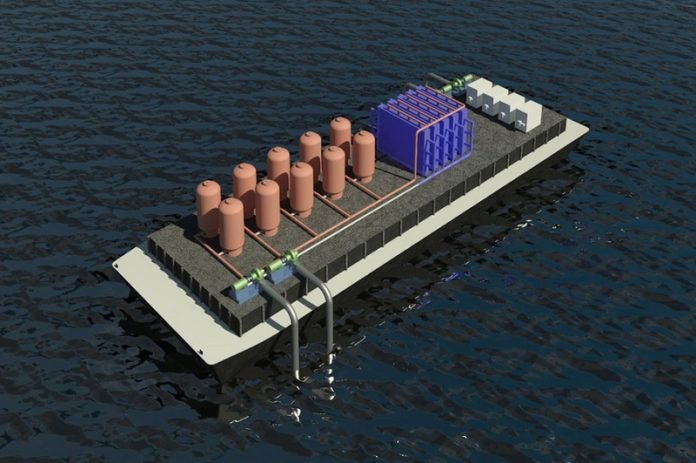
The system could also be implemented by ships that would process water as they travel, in order to help mitigate the significant contribution of ship traffic to overall emissions.
There are already international mandates to lower shipping’s emissions, and “this could help shipping companies offset some of their emissions, and turn ships into ocean scrubbers,” Varanasi says.
The system could also be implemented at locations such as offshore drilling platforms, or at aquaculture farms. Eventually, it could lead to a deployment of free-standing carbon removal plants distributed globally.
The process could be more efficient than air-capture systems, Hatton says, because the concentration of carbon dioxide in seawater is more than 100 times greater than it is in air. In direct air-capture systems it is first necessary to capture and concentrate the gas before recovering it.
“The oceans are large carbon sinks, however, so the capture step has already kind of been done for you,” he says. “There’s no capture step, only release.”
That means the volumes of material that need to be handled are much smaller, potentially simplifying the whole process and reducing the footprint requirements.
The research is continuing, with one goal being to find an alternative to the present step that requires a vacuum to remove the separated carbon dioxide from the water.
Another need is to identify operating strategies to prevent precipitation of minerals that can foul the electrodes in the alkalinization cell, an inherent issue that reduces the overall efficiency in all reported approaches.
Hatton notes that significant progress has been made on these issues, but that it is still too early to report on them. The team expects that the system could be ready for a practical demonstration project within about two years.
“The carbon dioxide problem is the defining problem of our life, of our existence,” Varanasi says. “So clearly, we need all the help we can get.”
The work was supported by ARPA-E.
Written by David L. Chandler.



