Sodium-ion batteries are a potential replacement for lithium batteries, but the anodes—positively charged electrodes—that work well for lithium-ion batteries don’t provide the same level of performance for sodium-ion batteries.
Amorphous carbon, which lacks a crystalline structure, is known to be a useful anode, because it has defects and voids that can be used to store sodium ions.
Nitrogen/phosphorus-doped carbon also offers appealing electrical properties.
In Applied Physics Reviews, researchers in China from Zhejiang University, Ningbo University, and Dongguan University of Technology describe how they applied basic physical concepts of atomic scale to build high-performance anodes for sodium-ion batteries.
“Recent studies have shown that doped amorphous carbon, especially electron-rich element-doped amorphous carbon, is a good anode for sodium storage,” said Tu.
“But there was no common explanation for how sodium storage works or the doping effect of doped carbon.”
On a quest for answers, the researchers used the concept of energy level orbitals to explain the affinity of pyrrolic nitrogen and a phosphorus-oxygen bond, their atomic interaction, electron distribution, and electron cloud configuration.
To get a closer look at distinct storage behavior, they applied first principles calculations, which is a method that uses basic physical quantities to calculate physical properties.
It is based on electron density function, a concept of quantum mechanics that can reveal a crystal’s molecular structure.
When they analyzed the electron distribution, system chemical parameters, and adsorption energies of sodium ions embedded within modified carbon materials, they found that pyrrolic nitrogen and phosphorus-oxygen bonds show real potential for sodium storage.
“Sodium ions tend to be stored within these two structures,” Tu said.
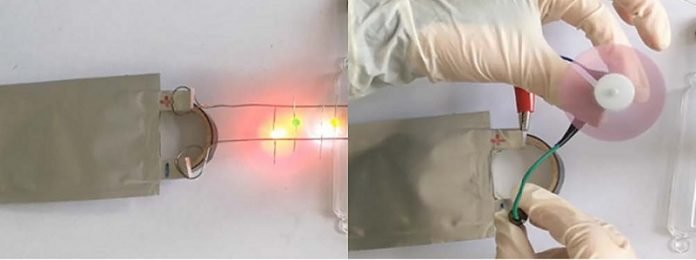
The researchers designed a hydrothermal treatment to build the precursor of a phosphorus-oxygen structure, then doped a carbon anode with the dual electron-rich elements. It shows “enhanced electrochemical performance in cycle life and capacity for batteries,” said Tu.
Their anode achieved a life cycle of 5,000 cycles, with an enhanced capacity of 220 milliampere hours/gram, and reduced capacity loss (0.003%/cycle).
“Our work fills the theoretical gap about the sodium storage behavior of electron-rich element-doped amorphous carbon and provides the experimental basis for using carbon,” said Tu.
“We provide directions to modify carbon materials for large-scale sodium-ion batteries.”



