It might look like a little game at the molecular scale.
Filament-like proteins in heart muscle cells have to be exactly the same length so that they can coordinate perfectly to make the heart beat.
Another protein decides when the filament is the right size and puts a wee little cap on it.
But, if that protein makes a mistake and puts the cap on too early, another protein, leiomodin, comes along and knocks the cap out of the way.
This little dance at the molecular scale might sound insignificant, but it plays a critical role in the development of healthy heart and other muscles.
Reporting in the journal, Plos Biology, a WSU research team has proven for the first time how the mechanism works.
The finding could someday lead to improved diagnostics and medical treatments for serious and sometimes devastating hereditary heart conditions that come about from genetic mutations in the proteins.
One of these conditions, cardiomyopathy, affects as many as one in 500 people around the world and can often be fatal or have lifetime health consequences.
A similar condition called nemaline myopathy affects skeletal muscles throughout the body with often devastating consequences.
“Mutations in these proteins are found in patients with myopathy,” said Alla Kostyukova, associate professor in the Gene and Linda Voiland School of Chemical Engineering and Bioengineering and leader of the project. “Our work is to prove that these mutations cause these problems and to propose strategies for treatment.”
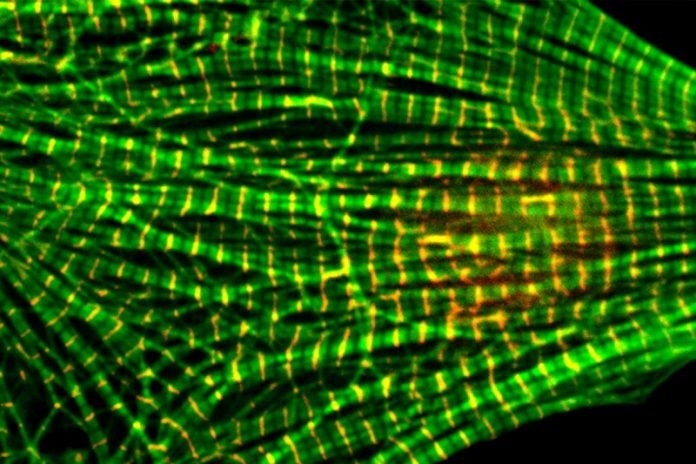
Heart muscle is made of tiny thick and thin filaments of proteins. With the help of electrical signals, the rope-like filaments bind and unbind in an intricate and precise architecture, allowing heart muscle to contract and beat.
The thin filaments are made of actin, the most abundant protein in the human body. Tropomysin, another protein, wraps itself around the actin filaments. Tropomyosin together with two other proteins, tropomodulin and leiomodin, at the end of the actin filaments act as a sort of cap and determine the filament length.
“It’s beautifully designed,” said Kostyukova, whose research is focused on understanding protein structures.
And, tightly regulated.
To keep heart muscle healthy, the actin filaments, which are about a micron long, all have to be the exact same length.
In families with cardiomyopathy, genetic mutations result in formation of filaments that are either too short or too long. Those affected can have significant heart problems that cause disability, illness and death.
In a project that spanned seven years, the researchers proved that leiomodin attaches to the end of the actin filament and kicks out the other protein, tropomodulin, to assure the actin filament’s proper length.
“This is the first time that this has been shown with the atomic-level precision,” said Dmitri Tolkatchev, research assistant professor in the Voiland School and lead author on the paper. “Previously, several laboratories attempted to solve this problem with very little success. With our data we finally have a direct proof.”
The researchers used state-of-the-art approaches to make the key proteins and study them at the molecular and cellular level. The work entailed designing the molecules, constructing them at the gene level in a plasmid, and then producing them into bacterial or cardiac cells.
The researchers used nuclear magnetic resonance, which works on the same physical principle as Magnetic Resonance Imaging (MRIs), to understand the proteins’ binding at the atomic level. They also used molecular dynamic simulation to model them.
“The probability of being able to show this mechanism was not high, but the impact of the discovery is,” said Tolkatchev, an expert in nuclear magnetic resonance. “This was a very important problem to study and could have a significant impact in the field of muscle mechanics.”
The researchers hope to continue the work, identifying additional components and molecular mechanisms that regulate thin filament architecture, whether diseased or healthy.
Written by Tina Hilding



