As businesses and schools tentatively reopen amid an ongoing pandemic, scientists at the Yale School of Public Health have developed a new method to determine whether someone has COVID-19.
The method, known as SalivaDirect, offers a range of benefits over existing testing methods and continues to draw widespread attention from around the United States and many countries beyond.
Here are answers to some of the most frequently asked questions.
What is SalivaDirect?
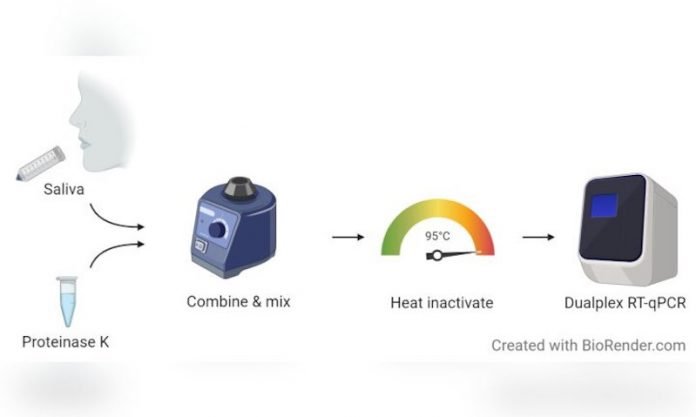
SalivaDirect is a new process developed at the Yale School of Public Health to test persons suspected of having COVID-19 for the virus, SARS-CoV-2.
It is ideal for large-scale testing and offers a number of advantages over traditional testing methods. SalivaDirect received emergency use authorization (EUA) from the U.S. Food and Drug Administration on Aug. 15, 2020, and is now trademarked.
Can I buy a SalivaDirect kit to test myself or my kids?
No. SalivaDirect is a complex protocol, or process, that requires a certified laboratory and trained technicians to conduct the testing.
It is not a kit, point-of-care or at-home rapid test and is not available commercially for purchase.
The Yale School of Public Health is looking into this and someday such kits may be available. However, this is months away at the earliest.
How much does a SalivaDirect test cost?
An exact price point has not yet been established. But the reagents (the substances used in chemical analysis) are inexpensive, costing anywhere between $1.29 to $4.30 per test under current pricing.
This cost is expected to further drop with large-volume purchases and the use of robotics for high-volume automated testing.
SalivaDirect also does not require expensive tubes containing special preservatives to collect your saliva sample, which further reduces costs.
How else is SalivaDirect different from other testing methods?
In addition to being less expensive, SalivaDirect offers a number of other advantages. They include:
It is non-invasive. SalivaDirect requires only a small sample of saliva as opposed to the standard nasopharyngeal (NP) swab.
The NP method requires what is essentially a long Q-tip that is inserted deep into the nostril and then rotated.
It is quick and safe. Because SalivaDirect only requires a small saliva sample, the time requirement for an individual is minimal, even less than a minute.
People basically have to spit into a small container. The procedure is safer in that there is less risk of exposure to health care workers collecting the samples.
It is accurate. Results show that SalivaDirect is highly sensitive and is accurate 94% of the time, comparable to results for NP-based tests.
My lab is also working on COVID-19. Can we use the SalivaDirect test?
Potentially yes. If your lab is CLIA certified and located within the United States it can apply for authorization to use SalivaDirect. Start by filling out this application form.
Will SalivaDirect be further improved in the coming weeks and months?
Yes. Everything can be improved, including SalivaDirect.
We are looking at ways to scale up automation and to make the test results even faster. A simplified and rapid test has the ability to be used by individuals in a point-of-care setting.
Can schools get their students tested with SalivaDirect? How about other organizations?
At the moment, unfortunately, we are currently not able to offer testing to the public, although we are working to develop testing partnerships that may be of use to you or your organization.
We will share information regarding these opportunities on our website as they become available.
Written by Michael Greenwood.



