In a new study, researchers have discovered a way to regenerate, in mice and human tissue, the cushion of cartilage found in joints.
They figured out how to regrow articular cartilage by first causing slight injury to the joint tissue, then using chemical signals to steer the growth of skeletal stem cells as the injuries heal.
The research was conducted by a team at the Stanford University School of Medicine.
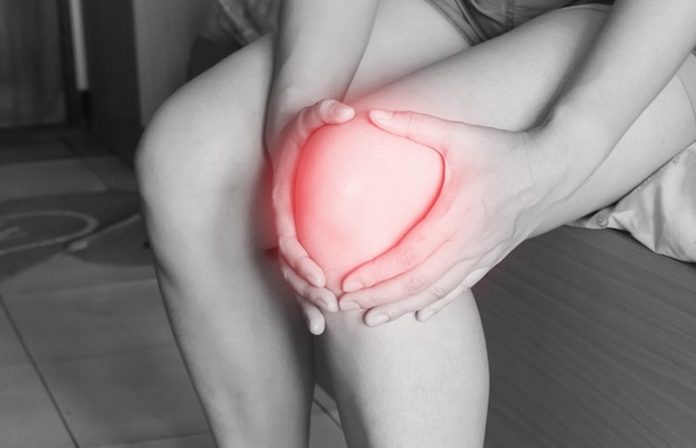
Loss of this slippery and shock-absorbing tissue layer, called articular cartilage, is responsible for many cases of joint pain and arthritis, which afflicts more than 55 million Americans.
Nearly one in four adult Americans suffer from arthritis, and far more are burdened by joint pain and inflammation generally.
Cartilage has practically zero regenerative potential in adulthood, so once it’s injured or gone, what we can do for patients has been very limited.
It’s extremely gratifying to find a way to help the body regrow this important tissue.
The work builds on previous research at Stanford that resulted in the isolation of the skeletal stem cell, a self-renewing cell that is also responsible for the production of bone, cartilage, and a special type of cell that helps blood cells develop in the bone marrow.
Articular cartilage is a complex and specialized tissue that provides a slick and bouncy cushion between bones at the joints.
When this cartilage is damaged by trauma, disease, or simply thins with age, bones can rub directly against each other, causing pain and inflammation, which can eventually result in arthritis.
Damaged cartilage can be treated through a technique called microfracture, in which tiny holes are drilled in the surface of a joint.
The microfracture technique prompts the body to create new tissue in the joint, but the new tissue is not much like cartilage.
Working in a mouse model, the team documented that microfracture could activate skeletal stem cells. Left to their own devices, however, those activated skeletal stem cells regenerated fibrocartilage in the joint.
But what if the healing process after microfracture could be steered toward the development of cartilage and away from fibrocartilage?
The researchers knew that as bone develops, cells must first go through a cartilage stage before turning into bone.
They had the idea that they might encourage the skeletal stem cells in the joint to start along a path toward becoming bone, but stop the process at the cartilage stage.
The researchers used a powerful molecule called bone morphogenetic protein 2 (BMP2) to initiate bone formation after microfracture, but then stopped the process midway with a molecule that blocked another signaling molecule important in bone formation, called vascular endothelial growth factor (VEGF).
What they ended up with was cartilage that is made of the same sort of cells as natural cartilage with comparable mechanical properties.
It also restored mobility to osteoarthritic mice and significantly reduced their pain.
As a proof of principle that this might also work in humans, the researchers transferred human tissue into mice that were bred to not reject the tissue and were able to show that human skeletal stem cells could be steered toward bone development but stopped at the cartilage stage.
The next stage of research is to conduct similar experiments in larger animals before starting human clinical trials.
The first human clinical trials might be for people who have arthritis in their fingers and toes.
One author of the study is an assistant professor of surgery Charles K.F. Chan, Ph.D.
The study is published in Nature Medicine.
Copyright © 2020 Knowridge Science Report. All rights reserved.