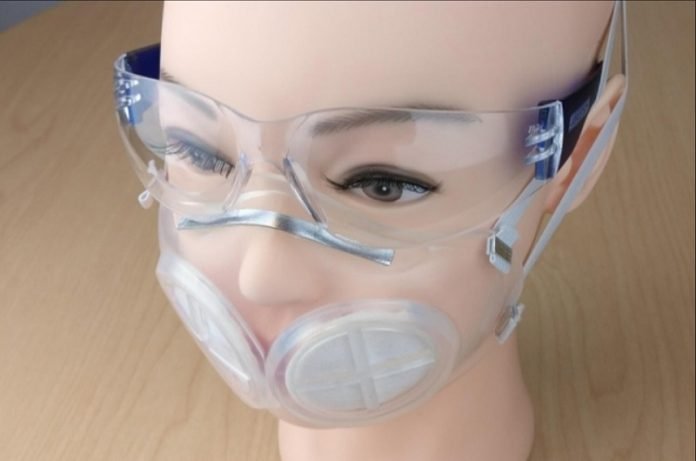
Researchers at MIT and Brigham and Women’s Hospital have designed a silicone rubber face mask that they believe could stop viral particles as effectively as N95 masks.
The masks are based on the shape of the 3M 1860 style of N95 masks normally used at Brigham and Women’s Hospital.
Most of the mask is silicone rubber, and there is space for one or two N95 filters, which are designed to be replaced after every use, while the rest of the mask can be sterilized and reused.
The prototype mask, which includes an N95 filter, can be easily sterilized and worn many times.
Researchers at MIT and Brigham and Women’s Hospital have designed a new face mask that they believe could stop viral particles as effectively as N95 masks.
Unlike N95 masks, the new masks were designed to be easily sterilized and used many times.
As the number of new Covid-19 cases in the United States continues to rise, there is still an urgent need for N95 masks for health care workers and others.
The new mask is made of durable silicone rubber and can be manufactured using injection molding, which is widely used in factories around the world. The mask also includes an N95 filter, but it requires much less N95 material than a traditional N95 mask.
“One of the key things we recognized early on was that in order to help meet the demand, we needed to really restrict ourselves to methods that could scale,” says Giovanni Traverso, an MIT assistant professor of mechanical engineering and a gastroenterologist at Brigham and Women’s Hospital.
“We also wanted to maximize the reusability of the system, and we wanted systems that could be sterilized in many different ways.”
The team is now working on a second version of the mask, based on feedback from health care workers, and is working to establish a company to support scaled-up production and seek approval from the FDA and the National Institute for Occupational Safety and Health (NIOSH).
Traverso is the senior author of a paper describing the new masks, which appears today in the British Medical Journal Open.
The lead authors of the study are James Byrne, a radiation oncologist at Brigham and Women’s Hospital and research affiliate at MIT’s Koch Institute for Integrative Cancer Research; Adam Wentworth, a research engineer at Brigham and Women’s Hospital and a research affiliate at the Koch Institute; Peter Chai, an emergency medicine physician at Brigham and Women’s Hospital; and Hen-Wei Huang, a research fellow at Brigham and Women’s Hospital and a postdoc at the Koch Institute.
Easy sterilization
The N95 masks that health care workers wear to protect against exposure to SARS-CoV-2 and other viruses are made from polypropylene fibers that are specially designed to filter out tiny viral particles.
Ideally, a health care worker would switch to a new mask each time they see a different patient, but shortages of these masks have forced doctors and nurses to wear them for longer than they are meant to be worn.
In recent months, many hospitals have begun sterilizing N95 masks with hydrogen peroxide vapor, which can be used up to 20 times on a single mask.
However, this process requires specialized equipment that is not available everywhere, and even with this process, one mask can be worn for only a single day.
The MIT/BWH team set out to design a mask that could be safely sterilized and reused many times. They decide on silicone rubber — the material that goes into silicone baking sheets, among other products — because it is so durable.
Liquid silicone rubber can be easily molded into any shape using injection molding, a highly automated process that generates products rapidly.
The masks are based on the shape of the 3M 1860 style of N95 masks, the type normally used at Brigham and Women’s Hospital. Most of the mask is made of silicone rubber, and there is also space for one or two N95 filters.
Those filters are designed to be replaced after every use, while the rest of the mask can be sterilized and reused.
“With this design, the filters can be popped in and then thrown away after use, and you’re throwing away a lot less material than an N95 mask,” Wentworth says.
The researchers tested several different sterilization methods on the silicone masks, including running them through an autoclave (steam sterilizer), putting them in an oven, and soaking them in bleach and in isopropyl alcohol. They found that after sterilization, the silicone material was undamaged.
Fit test
To test the comfort and fit of the masks, the researchers recruited about 20 health care workers from the emergency department and an oncology clinic at Brigham and Women’s Hospital.
They had each of the subjects perform the standard fit test that is required by the Occupational Safety and Health Administration (OSHA) for N95 masks.
During this test, the subject puts the mask on and then performs a series of movements to see if the mask stays in place. A nebulized sugar solution is sprayed in the room, and if the subject can taste or smell it, it means the mask is not properly fitted.
All 20 subjects passed the fit test, and they reported that they were able to successfully insert and remove the N95 filter.
When asked their preference between the new mask, a typical N95 mask, and a standard surgical mask, most either said they had no preference or preferred the new silicone mask, Byrne says. They also gave the new mask high ratings for fit and breathability.
The researchers are now working on a second version of the mask, which they hope to make more comfortable and durable. They also plan to do additional lab tests measuring the masks’ ability to filter viral particles.
As many regions of the United States have seen a surge in Covid-19 cases over the past month, hospitals in those areas face the possibility of mask shortages. There is also a need for more masks in parts of the world that don’t have the equipment needed for hydrogen peroxide sterilization.
“We know that Covid is really not going away until a vaccine is prevalent,” Byrne says. “I think there’s always going to be a need for masks, whether it be in the health care setting or in the general public.”
The research was funded, in part, by the Prostate Cancer Foundation, the MIT Department of Mechanical Engineering, Brigham and Women’s Hospital, the National Institutes of Health, E-Ink Corporation, Gilead Sciences, Philips Biosensing, and the Hans and Mavis Lopater Psychosocial Foundation.
Written by Anne Trafton.



