The more we learn about the novel coronavirus (COVID-19), the more unknowns seem to arise.
These ever-emerging mysteries highlight the desperate need for more data to help researchers and physicians better understand—and treat—the extremely contagious and deadly disease.
Researchers at Northwestern University and Shirley Ryan AbilityLab in Chicago have developed a novel wearable device and are creating a set of data algorithms specifically tailored to catch early signs and symptoms associated with COVID-19 and to monitor patients as the illness progresses.
Capable of being worn 24/7, the device produces continuous streams of data and uses artificial intelligence to uncover subtle, but potentially life-saving, insights.
Filling a vital data gap, it continuously measures and interprets coughing and respiratory activity in ways that are impossible with traditional monitoring systems.
Developed in an engineering laboratory at Northwestern and using custom algorithms being created by Shirley Ryan AbilityLab scientists, the devices are currently being used at Shirley Ryan AbilityLab by COVID-19 patients and the healthcare workers who treat them.
About 25 affected individuals began using the devices two weeks ago. They are being monitored both in the clinic and at home, totaling more than 1,500 cumulative hours and generating more than one terabyte of data.
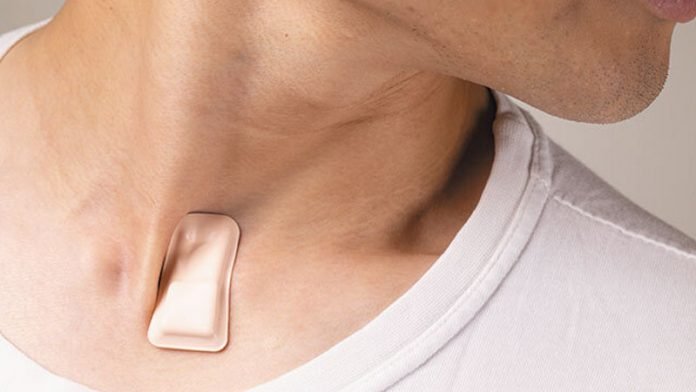
About the size of a postage stamp, the soft, flexible, wireless, thin device sits just below the suprasternal notch—the visible dip at the base of the throat.
From this location, the device monitors coughing intensity and patterns, chest wall movements (which indicate labored or irregular breathing), respiratory sounds, heart rate and body temperature, including fever.
From there, it wirelessly transmits data to a HIPAA-protected cloud, where automated algorithms produce graphical summaries tailored to facilitate rapid, remote monitoring.
“The most recent studies published in the Journal of the American Medical Association suggest that the earliest signs of a COVID-19 infection are fever, coughing and difficulty in breathing.
Our device sits at the perfect location on the body—the suprasternal notch—to measure respiratory rate, sounds and activity because that’s where airflow occurs near the surface of the skin,” said Northwestern’s John A. Rogers, who led the technology development.
“We developed customized devices, data algorithms, user interfaces and cloud-based data systems in direct response to specific needs brought to us by frontline healthcare workers.
We’re fully engaged in contributing our expertise in bioelectronic engineering to help address the pandemic, using technologies that we are able to deploy now, for immediate use on actual patients and other affected individuals.
The measurement capabilities are unique to this device platform—they cannot be accomplished using traditional watch or ring-style wearables that mount on the wrist or the finger.”
“We anticipate that the advanced algorithms we are developing will extract COVID-like signs and symptoms from the raw data insights and symptoms even before individuals may perceive them,” said Arun Jayaraman, a research scientist at Shirley Ryan AbilityLab, who is leading the algorithm development.
“These sensors have the potential to unlock information that will protect frontline medical workers and patients alike—informing interventions in a timely manner to reduce the risk of transmission and increase the likelihood of better outcomes.”
Continuous monitoring from hospital to home
The mysterious ways that COVID-19 affects the body seem to get stranger and stranger.
Many patients’ symptoms fully disappear before they suddenly and unexpectedly begin deteriorating—sometimes within a matter of hours. Other patients have recovered and tested “negative” but later test “positive” again.
The unknowns underscore the need for continuous patient monitoring to ensure that physicians can intervene at the slightest sign of trouble.
The Northwestern and Shirley Ryan AbilityLab teams’ device provides around-the-clock monitoring for COVID-19 patients and those exposed to them.
“Having the ability to monitor ourselves and our patients—and being alerted to changing conditions in real time—will give clinicians a new and important tool in the fight against COVID-19,” said Dr. Mark Huang, a physician at Shirley Ryan AbilityLab, who has worn the sensor.
“The sensor also will offer clinicians and patients peace of mind as it monitors COVID-like symptoms, potentially prompting earlier intervention and treatment.”
The device can monitor hospitalized patients and then be taken home to continue 24/7 supervision.
The real-time data streaming from patients gives insights into their health and outcomes that is currently not being captured or analyzed by traditional monitoring systems.
“Nobody has ever collected this type of data before,” Rogers said. “Earlier detection is always better and our devices provide important and unique capabilities in that context.
For patients who have contracted the disease, the value is even more clear, as the data represent quantitative information on respiratory behavior, as a mechanism to track the progression and/or the effects of treatments.”
“This opens up new telemedicine strategies as we won’t have to bring in patients for monitoring,” Jayaraman said.
“Physicians can potentially review the patients’ data for hours, days or weeks, immediately through a customized graphical user interface to a cloud data management system that is being set up for this purpose, to see an overall image of how the patient is doing.”
Although the wearable device is currently unable to measure blood oxygenation levels, which is an important component of lung health, the team plans to incorporate this capability in its next round of devices.
The Rogers lab has already suc … ntensive care units. Rogers believes they can easily apply that research to the COVID-tailored devices.
Warning system for the most at-risk
Not only can the device monitor the progress of COVID-19 patients, it could also provide early warning signals to the frontline workers who are most at risk for catching this remarkably infectious disease.
The device offers the potential to identify symptoms and to pick up trends before the workers notice them, thereby providing an opportunity to engage in appropriate precautionary measures and to seek further testing as quickly as possible.
“People with obvious, severe symptoms are going to the hospital, being tested or being told to self-isolate,” Jayaraman said.
“For those who have symptoms they perceive as mild or seasonal allergies, there is no warning system. They could be in contact with others and unknowingly spread infection.”
Assessing efficacy of new therapeutics
As researchers rush for a COVID-19 cure, physicians have been trying exploratory, sometimes unproven, treatments to help their patients. This is another area where Rogers’ and Jayaraman’s device can play a role.
“Early reports of certain proposed treatments suggest that they can eliminate coughing symptoms more quickly than a placebo,” Rogers said.
“Nobody, however, is quantifying certain key symptoms, such as coughing—duration, frequency, amplitude, sounds, etc.
Our device allows for precision measurement of this essential, yet currently unquantified, aspect of the disease.”
In the future, this sensor package could help researchers and physicians quantify which therapeutics are working best.
“At the simplest level, our systems allow assessments based on data, in a quantitative way, without relying on human judgment of whether a patient is coughing more or less,” Rogers said.
Device initially conceived for stroke patients
The new device builds on recent research from a collaboration between Rogers’ and Jayaraman’s labs, first published on the cover of the February 2020 issue of Nature Biomedical Engineering, with a focus on monitoring swallowing and speech disorders in patients recovering from stroke.
These sensors work by precisely measuring vibratory signatures from the throat and chest. By measuring vibrations rather than acoustics, the team avoids noise from background sounds and it bypasses privacy issues.
In response to requests and inquiries from the medical community, Rogers and Jayaraman realized they could use this technology to measure the vibratory signatures of COVID-like symptoms, including chest wall movements and cough.
Jayaraman’s team is developing custom signal processing and machine-learning algorithms to train the device how to recognize coughs in the data.
“As the algorithm becomes smarter, our hope is that it will begin to discriminate among which coughs are COVID-like and which are from something more benign,” Jayaraman said.
“The most basic approach, already deployed on COVID-19 patients and health care workers, simply counts coughs and their intensity.”
More advanced analytics packages will be available within the next few weeks.
Bypassing already-stressed supply chains
Thanks to a generous gift from Northwestern University trustees Kimberly K. Querrey and Louis A. Simpson, Rogers and his team are able to respond quickly to requests for devices. Leveraging a set of manufacturing tools available in the
new Simpson Querrey Biomedical Research Building in Chicago, the team is already producing dozens of devices per week.
Rogers estimates that his team could produce up to hundreds of devices per week—all in house, largely bypassing the need for external vendors and complex supply chains.
“Quickly developing new technologies internally has never been more crucial,” Querrey said.
“This work proves the power of STEM and why it’s so critical to the University and beyond to have world-class researchers like John.
I am so proud of John and his team, while working remotely, for thinking outside the box and using their collaborations to help protect our healthcare workers.
We are excited to be able to develop these devices within the University and get them in the hands of those needing them most. T
he ability to measure vibratory signatures could really help with early detection of COVID-19.”
“This crucial philanthropic support has allowed us to develop and deploy the devices and an associated software infrastructure almost immediately, within days, after we started receiving requests from the medical community—without waiting for external vendors, most of which are mostly shut down with the stay-at-home orders,” Rogers said.
“In this way, we avoid already-stressed supply chains. We just do it ourselves.”
Comfortable and easy to use
In mid-March, Kelly McKenzie felt foggy and developed a low-grade headache. Having recently returned from a work-related trip overseas, she assumed it was jetlag.
But as her symptoms progressed to include cough and congestion, she started to worry. Although her symptoms were not severe enough to seek COVID-19 testing, she knew she should self-isolate.
“Between my international travel and the symptoms, my director and I decided it was best for me to stay home from work, so I wasn’t bringing anything contagious into the hospital,” said McKenzie, who is a research physical therapist at Shirley Ryan AbilityLab.
McKenzie joined the pilot study to test the device and train the algorithm with her symptoms.
After wearing the sensor around the clock for a week, she was amazed by the comfort of the soft silicone material and ease of use.
Wearers simply charge the device, put it on and it immediately begins to work—streaming real-time data to a smartphone or tablet.
“When you first put it on, you can feel it just because it’s new and different,” McKenzie said. “But after you have worn it for a while, you don’t even notice it.”
Because it is fully encased without wires, electrodes, charge ports or removable batteries, the device can be worn while exercising or in the shower. It turns out this also is important for sterilization and reuse.
“This is absolutely critical for use in the context of this extremely contagious disease,” Rogers said.
“Because it is fully sealed in a soft biocompatible silicone material, it can be completely immersed in alcohol, and then exposed to a gas-based system for rigorous sterilization.
If there were exposed regions, or plugs or ports or other physical interfaces, the device would not be relevant for this application.”
What’s next?
In the coming weeks, the Northwestern and Shirley Ryan AbilityLab teams will continue collecting patient data to strengthen their algorithms—through deployments both in the clinic and at home.
They also are responding to other requests for access to the technology, across the medical complex in Chicago. Additional deployments are starting now.
Rogers and Jayaraman also are examining data from patients recovering from COVID-19, attempting to determine when they are no longer contagious.
Some of the patients wearing the device have been dismissed from the acute-care hospital and are rehabilitating at Shirley Ryan AbilityLab.
In the future, this device could help determine whether post-COVID patients still have minor, perhaps imperceptible symptoms.
Rogers hopes the device will not just tell physicians how to best treat COVID-19 but also inform researchers about the nature of the virus itself.
“The growing amount of information and understanding around COVID-19 as a disease will be critically important to containing and treating the current outbreak as well as those that might occur in the future,” he said.
“We hope, and we believe, that these devices may help in these efforts by identifying and quantifying characteristics and essential features of cough and respiratory activity associated with this disease.”
To accelerate the deployment of this device, the team recently launched a lean engineering-centric company, Sonica Health, based on intellectual property jointly developed by Northwestern and the Shirley Ryan AbilityLab and licensed through Northwestern’s Innovation and New Ventures Office.
Exploring use of the device for the COVID-19 response is supported by the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services.
BARDA invests in the innovation, advanced research and development, acquisition and manufacturing of medical countermeasures—vaccines, drugs, therapeutics, diagnostic tools and non-pharmaceutical products needed to combat health security threats.
To date, 54 BARDA-supported products have achieved regulatory approval, licensure or clearance.
DRIVe (Division of Research, Innovation and Ventures) within BARDA, catalyzes the development of innovative products and approaches, like the Sonica Health technology, with the aim of solving major health security challenges.
Written by Amanda Morris.



