A research team has produced a super-stable artificial protein ball that apparently defies the rules of geometry and which may have applications in materials science and medicine.
Researchers are interested in making artificial protein cages in the hope that they can design them to have useful properties not found in nature.
There are two challenges to achieving this goal.
The first is the geometry problem: some proteins may have great potential utility but have the wrong shape to assemble into cages.
The second problem is complexity: in nature, many proteins that form a protein cage are held together by a complex network of chemical bonds and these are very difficult to predict and simulate.
In new work, published in Nature, researchers found a way to solve both of these problems.
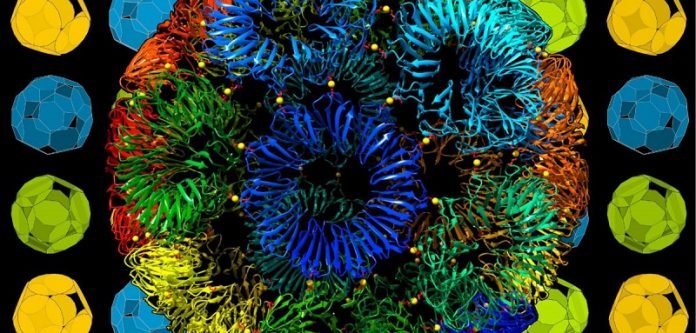
Professor Heddle, senior author of the research, said: ‘We were able to replace the complex interactions between proteins with a simple ‘staple’ consisting of a single gold atom.
This simplifies the design problem and allows us to imbue the cages with new properties such as assembly and disassembly on demand.’
The research has also found a way to get around the geometrical problem: the building block of a protein cage is an 11-sided shape.
Theoretically, this should not be able to form the faces of a regular convex polyhedron.
However, the research has found that while this is mathematically true, some so-called ‘impossible shapes’ can assemble into cages which are so close to being regular that the errors are not noticeable.
Central to the study was the ability to characterize different cages, as well the ability to monitor and thereby understand the (dis)assembly dynamically.
This work was done in the groups of Professors Justin Benesch and Philipp Kukura at Oxford, using innovative mass measurement approaches with a particular focus on biomolecular structure and assembly.
Justin Benesch, in the Department of Chemistry, said: ‘The ability to interrogate the cages using the advanced mass measurement approaches we have developed here in Oxford, both on the level of their assembly and the constituent building block, was key to not just validating their structure, but also the mechanism by which they are formed.’
The potential implications of the work are far-reaching. The researchers hope that the work can be expanded further to produce cages with new structures and new capabilities with potential applications particularly in drug delivery.
The research ‘An ultra-stable gold-coordinated protein cage displaying reversible assembly’ is published in Nature.



