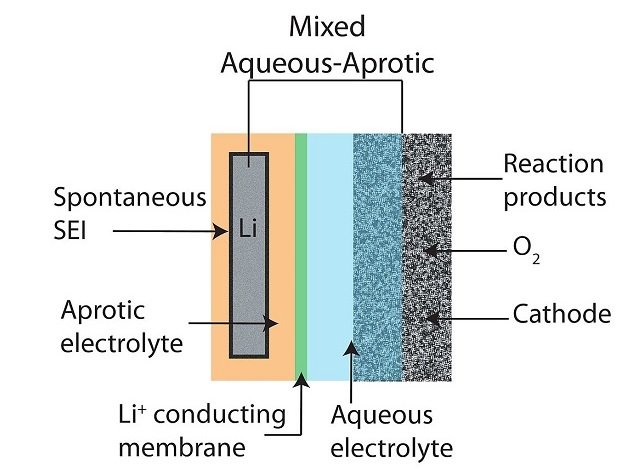
Lithium-air battery, Li-air battery for short, is a renewable energy source. It is considered to play an important role in energy storage to meet the increasing demand for global energy use.
Li-air battery was originally proposed in the 1970s as a power source for electric vehicles. In late 2000s, it captured scientists’ attention due to the development of material technology.
However, several technical challenges constrain the use of Li-air battery. One is appropriate cathode catalyst to enhance the electrode reactions in the battery.
In a recent study published in Journal of The Electrochemical Society, researchers from Northeastern University and US Army Power Division found a new way to advance Li-air battery with novel catalyst.
In the study, researchers examined the electrochemical activity of a Li-air battery cathode catalyst from a specific lithium rich metal oxide. The catalyst was composed of three metals, including nickel, manganese, and cobalt.
The novel catalyst could enhance two electrode reactions in a Li-air cell. One reaction was oxygen reduction reaction, and the other was oxygen evolution reaction.
The manganese played an important role in the catalysis of the oxygen reduction reaction, and the cobalt catalyzed the charge reaction of the Li-air battery.
Researchers found that the novel catalyst had better electrochemical activity, e.g., the initial Li-air produced from the reactions was more stable. In addition, the catalyzed battery cells had longer cycle life than uncatalyzed cells under similar cycling conditions.
Importantly, the Li-rich metal oxides can also be used as cathodes for Li-ion battery, the current finding provides a possibility that both Li-air and Li-ion battery can be used sustainably.
This is a good news for electric vehicle industry, which is interested in batteries with high energy density.
It also can advance the storage of solar battery. Up to 20% of the solar power produced by conventional solar cells is lost as it travels to and charges a battery. A hybrid solar cell for 100% grid backup has been developed recently, and the latest device uses Li-air battery.
Citation: Ates MN, et al. (2016). In Situ Formed Layered-Layered Metal Oxide as Bifunctional Catalyst for Li-Air Batteries, Journal of The Electrochemical Society, 163: A2464-A2474. doi: 10.1149/2.0111613jes
Figure legend: This Knowridge.com image is credited to Na9234.